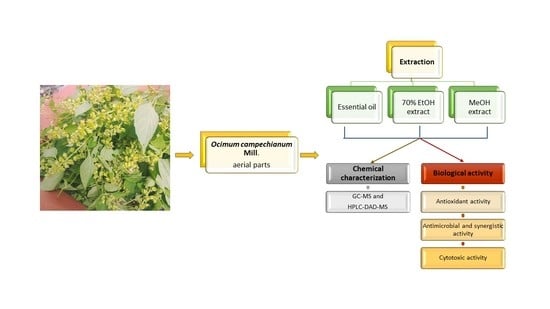
Ocimum campechianum Mill. from Amazonian Ecuador: Chemical Composition and Biological Activities of Extracts and Their Main Constituents (Eugenol and Rosmarinic Acid)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Characterization
2.2. Antioxidant Activity
2.3. Antimicrobial Activity of O. campechianum
2.4. Cytotoxic Activity of O. campechianum
2.5. Mutagenic Assay (Ames Test)
3. Materials and Methods
3.1. Plant Material
3.2. Preparation of Extracts
3.3. Gas Chromatography Coupled to Mass Spectrometric and Flame Ionization Analyses
3.4. HPLC-DAD-MS Analysis
3.5. DPPH Scavenging Activity
3.6. ABTS Scavenging Activity
3.7. Antibacterial Activity
3.8. Antimicrobial Activity against Candida spp. and Synergy Test
3.8.1. Cell Lines and Culture Conditions
3.8.2. Cell Viability Assay
3.9. Mutagenic Assay
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Sample Availability
Acknowledgments
Conflicts of Interest
References
- Bailon-Moscoso, N.; Romero-Benavides, J.C.; Tinitana-Imaicela, F.; Ostrosky-Wegman, P. Medicinal plants of Ecuador: A review of plants with anticancer potential and their chemical composition. Med. Chem. Res. 2015, 24, 2283–2296. [Google Scholar] [CrossRef]
- Worldatlas. 17 Most Ecologically Diverse Countries on Earth. Available online: http://www.worldatlas.com/articles/ecologically-megadiverse-countries-of-the-world.html (accessed on 2 December 2020).
- Guerrini, A.; Sacchetti, G.; Grandini, A.; Spagnoletti, A.; Asanza, M.; Scalvenzi, L. Cytotoxic Effect and TLC Bioautography-Guided Approach to Detect Health Properties of Amazonian Hedyosmum sprucei Essential Oil. Evid. Based Complement. Alternat. Med. 2016, 2016, 1638342. [Google Scholar] [CrossRef] [Green Version]
- Lubbe, A.; Verpoorte, R. Cultivation of medicinal and aromatic plants for specialty industrial materials. Ind. Crops Prod. 2011, 34, 785–801. [Google Scholar] [CrossRef]
- Maia, J.G.S.; Andrade, E.H.A. Database of the Amazon aromatic plants and their essential oils. Quím. Nova 2009, 32, 595–622. [Google Scholar] [CrossRef] [Green Version]
- Sacchetti, G.; Guerrini, A.; Noriega, P.; Bianchi, A.; Bruni, R. Essential oil of wild Ocotea quixos (Lam.) Kosterm. (Lauraceae) leaves from Amazonian Ecuador. Flavour Frag. J. 2006, 21, 674–676. [Google Scholar] [CrossRef]
- Malagón, O.; Ramírez, J.; Andrade, J.M.; Morocho, V.; Armijos, C.; Gilardoni, G. Phytochemistry and ethnopharmacology of the Ecuadorian flora. A Review. Nat. Prod. Commun. 2016, 11, 297–314. [Google Scholar] [CrossRef] [Green Version]
- Batish, D.R.; Singh, H.P.; Kohli, R.K.; Kaur, S. Eucalyptus essential oil as a natural pesticide. For. Ecol. Manag. 2008, 256, 2166–2174. [Google Scholar] [CrossRef]
- Caballero-Serrano, V.; McLaren, B.; Carrasco, J.C.; Alday, J.G.; Fiallos, L.; Amigo, J.; Onaindia, M. Traditional ecological knowledge and medicinal plant diversity in Ecuadorian Amazon home garden. Glob. Ecol. Conserv. 2019, 17, e00524. [Google Scholar] [CrossRef]
- Scalvenzi, L.; Radice, M.; Toma, L.; Severini, F.; Boccolini, D.; Bella, A.; Guerrini, A.; Tacchini, M.; Sacchetti, G.; Chiurato, M.; et al. Larvicidal activity of Ocimum campechianum, Ocotea quixos and Piper aduncum essential oils against Aedes aegypti. Parasite 2019, 26, 23. [Google Scholar] [CrossRef] [Green Version]
- Ballesteros, J.L.; Tacchini, M.; Spagnoletti, A.; Grandini, A.; Paganetto, G.; Neri, L.M.; Marengo, A.; Angiolella, L.; Guerrini, A.; Sacchetti, G. Rediscovering Medicinal Amazonian Aromatic Plants: Piper carpunya (Piperaceae) Essential Oil as Paradigmatic Study. Evid. Based Complement. Alternat. Med. 2019, 2019, 6194640. [Google Scholar] [CrossRef]
- Guerrini, A.; Rossi, D.; Grandini, A.; Scalvenzi, L.; Rivera, P.F.N.; Andreotti, E.; Sacchetti, G. Biological and chemo-diverse characterization of Amazonian (Ecuador) Citrus petitgrains. J. Appl. Bot. Food Qual. 2014, 87, 108–116. [Google Scholar]
- Rossi, D.; Guerrini, A.; Maietti, S.; Bruni, R.; Paganetto, G.; Poli, F.; Sacchetti, G. Chemical fingerprinting and bioactivity of Amazonian Ecuador Croton lechleri Müll. Arg. (Euphorbiaceae) stem bark essential oil: A new functional food ingredient? Food Chem. 2011, 126, 837–848. [Google Scholar] [CrossRef]
- Scalvenzi, L.; Grandini, A.; Spagnoletti, A.; Tacchini, M.; Neill, D.; Ballesteros, J.L.; Sacchetti, G.; Guerrini, A. Myrcia splendens (Sw.) DC. (syn. M. fallax (Rich.) DC.) (Myrtaceace) essential oil from Amazonian Ecuador: A chemical characterization and bioactive profile. Molecules 2017, 22, 1163. [Google Scholar] [CrossRef] [Green Version]
- Jaramillo, B.; Duarte, E.; Delgado, W. Bioactivity of essential oil from Ocimum micranthum Willd, collected from Bolivar department, Colombia. Rev. Cuba. Plantas Med. 2014, 19, 185–196. [Google Scholar]
- Salles Trevisan, M.T.; de Vasconelos Silva, M.G.; Pfundstein, B.; Spiegelhalder, B.; Wyn Owen, R. Characterization of the Volatile Pattern and Antioxidant Capacity of Essential Oils from Different Species of the Genus Ocimum. J. Agric. Food Chem. 2006, 54, 4378–4382. [Google Scholar] [CrossRef]
- De Vasconcelos Silva, M.G.; Abreu Matos, F.J.; Lacerda Machado, M.I.; de Oliveira Silva, F. Essential Oil Composition of the Leaves of Ocimum micranthum Willd. J. Essent. Oil Res. 2004, 16, 189–190. [Google Scholar] [CrossRef]
- Sacchetti, G.; Medici, A.; Maietti, S.; Radice, M.; Muzzoli, M.; Manfredini, S.; Braccioli, E.; Bruni, A. Composition and Functional Properties of the Essential Oil of Amazonian Basil, Ocimum micranthum Willd., Labiatae in Comparison with Commercial Essential Oils. J. Agric. Food. Chem. 2004, 52, 3486–3491. [Google Scholar] [CrossRef]
- Ruiz-Vargas, J.A.; Morales-Ferra, D.L.; Ramirez-Avila, G.; Zamilpa, A.; Negrete-Leon, E.; Acevedo-Fernandez, J.J.; Pena-Rodriguez, L.M. α- Glucosidase inhibitory activity and in vivo antihyperglycemic effect of secondary metabolites from the leaf infusion of Ocimum campechianum Mill. J. Ethnopharm. 2019, 243, 112081. [Google Scholar] [CrossRef]
- Gry, J.; Black, L.; Damsted Eriksen, F.; Pilegaard, K.; Plumb, J.; Rhodes, M.; Sheehan, D.; Kiely, M.; Kroon, P.A. EuroFIR-BASIS–a combined composition and biological activity database for bioactive compounds in plant-based foods. Trends Food. Sci. Tech. 2007, 18, 434–444. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Busines Media: Carol Stream, IL, USA, 2007. [Google Scholar]
- Figueiredo, P.L.B.; Silva, S.G.; Nascimento, L.D.; Ramos, A.R.; Setzer, W.N.; da Silva, J.K.R.; Andrade, E.H.A. Seasonal Study of Methyleugenol Chemotype of Ocimum campechianum Essential Oil and Its Fungicidal and Antioxidant Activities. Nat. Prod. Commun. 2018, 13, 1055–1058. [Google Scholar] [CrossRef] [Green Version]
- Villalva, M.; Jaime, L.; Aguado, E.; Nieto, J.A.; Reglero, G.; Santoyo, S. Anti-Inflammatory and Antioxidant Activities from the Basolateral Fraction of Caco-2 Cells Exposed to a Rosmarinic Acid Enriched Extract. J. Agric. Food Chem. 2018, 66, 1167–1174. [Google Scholar] [CrossRef]
- Vinueza, D.; Yanza, K.; Tacchini, M.; Grandini, A.; Sacchetti, G.; Chiurato, M.; Guerrini, A. Flavonoids in Ecuadorian Oreocallis grandiflora (Lam.) R. Br.: Perspectives of use of this species as a food supplement. Evid. Based Complement. Alternat. Med. 2018, 2018, 1353129. [Google Scholar] [CrossRef]
- Kwee, E.M.; Niemeyer, E.D. Variations in phenolic composition and antioxidant properties among 15 basil (Ocimum basilicum L.) cultivars. Food Chem. 2011, 128, 1044–1050. [Google Scholar] [CrossRef]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant Activity of Essential Oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef]
- Kamatou, G.P.; Vermaak, I.; Viljoen, A.M. Eugenol--from the remote Maluku Islands to the international market place: A review of a remarkable and versatile molecule. Molecules 2012, 17, 6953–6981. [Google Scholar] [CrossRef]
- De Araújo Lopes, A.; da Fonseca, F.N.; Rocha, T.M.; de Freitas, L.B.; Oliveira Araújo, E.V.; Tenazoa Wong, D.V.; Pereira Lima Júnior, R.C.; Almeida Moreira, L.K. Eugenol as a promising molecule for the treatment of dermatitis: Antioxidant and anti-inflammatory activities and its nanoformulation. Oxid. Med. Cell Longev. 2018, 2018, 8194849. [Google Scholar] [CrossRef] [Green Version]
- Valero, D.; Valverde, J.M.; Martinez-Romero, D.; Guillen, F.; Castillo, S.; Serrano, M. The combination of modified atmosphere ackaging with eugenol or thymol to maintain quality, safety and functional properties of table grapes. Postharvest Biol. Technol. 2006, 41, 317–327. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wang, S.Y.; Yin, J.-J.; Parry, J.; Yu, L.L. Enhancing antioxidant, antiproliferation, and free radical scavenging activities in strawberries with essential oils. J. Agric. Food Chem. 2007, 55, 6527–6532. [Google Scholar] [CrossRef]
- Jin, P.; Wu, X.; Xu, F.; Wang, X.; Wang, J.; Zheng, Y. Enhancing antioxidant capacity and reducing decay of Chinese bayberries by essential oils. J. Agric. Food Chem. 2012, 60, 3769–3775. [Google Scholar] [CrossRef]
- Nadeem, M.; Imran, M.; Gondal, T.A.; Imran, A.; Shahbaz, M.; Amir, R.; Sajid, M.W.; Qaisrani, T.; Atif, M.; Hussain, G.; et al. Therapeutic potential of rosmarinic acid: A comprehensive review. Appl. Sci. 2019, 9, 3139. [Google Scholar] [CrossRef] [Green Version]
- Commission Implementing Regulation (EU). Commission Implementing Regulation (EU) No 546/2013 Approving the Active Substance Eugenol, in Accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council Concerning the Placing of Plant Protection Products on the Market, and Amending the Annex to Commission Implementing Regulation (EU) No 540/2011. OJ L 2013, 163, 17–20. [Google Scholar]
- Commission Implementing Regulation (EU). Commission Implementing Regulation (EU) No 540/2011implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as Regards the List of Approved Active Substances Text with EEA Relevance. OJ L 2011, 153, 1–186. [Google Scholar]
- Caamal-Herrera, I.O.; Carrillo-Cocom, L.M.; Escalante-Réndiz, D.Y.; Aráiz-Hernández, D.; Azamar-Barrios, J.A. Antimicrobial and antiproliferative activity of essential oil, aqueous and ethanolic extracts of Ocimum micranthum Willd leaves. BMC Complement. Altern. Med. 2018, 18, 55. [Google Scholar] [CrossRef] [Green Version]
- Zaidi, K.U.; Shah, F.; Parmar, R.; Thawani, V. Anticandidal synergistic activity of Ocimum sanctum and fluconazole of azole resistance strains of clinical isolates. J. Mycol. Med. 2018, 28, 289–293. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Fangjun, L.; Zhijia, Y. Tumor suppressive roles of eugenol in human lung cancer cells. Thorac. Cancer. 2018, 9, 25–29. [Google Scholar] [CrossRef]
- Cheng, Z.; Moore, J.; Yu, L.L. High-Throughput Relative DPPH Radical Scavenging Capacity Assay. J. Agric. Food Chem. 2006, 54, 7429–7436. [Google Scholar] [CrossRef]
- Horszwald, A.; Andlauer, W. Characterisation of bioactive compounds in berry juices by traditional photometric and modern microplate methods. J. Berry Res 2011, 1, 189–199. [Google Scholar] [CrossRef] [Green Version]
- CLSI (Clinical Laboratory Standards Institute). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 9th ed.; Approved Standard M07-A10; CLSI: Wayne, PA, USA, 2015. [Google Scholar]

| No. | Component 1 | Area% 2 | AI exp 3 | AI lit 4 |
|---|---|---|---|---|
| 1 | α-pinene | 0.3 | 929 | 932 |
| 2 | camphene | 0.1 | 944 | 946 |
| 3 | sabinene | 0.8 | 967 | 969 |
| 4 | β-pinene | 1.2 | 973 | 974 |
| 5 | myrcene | 2.3 | 987 | 988 |
| 6 | 1,8-cineole | 4.4 | 1028 | 1026 |
| 7 | cis-β-ocimene | 1.3 | 1030 | 1032 |
| 8 | trans-ocimene | 0.4 | 1046 | 1044 |
| 9 | linalool | 1.6 | 1101 | 1095 |
| 11 | allo-ocimene | 1.7 | 1126 | 1128 |
| 12 | mentha-1,5 dien-8 ol | 0.3 | 1170 | 1166 |
| 13 | α-terpineol | 0.4 | 1189 | 1186 |
| 14 | neral | 0.1 | 1238 | 1235 |
| 15 | δ-elemene | 4.2 | 1338 | 1335 |
| 16 | eugenol | 43.6 | 1359 | 1356 |
| 17 | α-copaene | 0.5 | 137 | 1374 |
| 19 | β-elemene | 8.1 | 1388 | 1389 |
| 20 | β-caryophyllene | 10.8 | 1410 | 1416 |
| 21 | γ-elemene | 0.3 | 1427 | 1434 |
| 22 | trans-α-bergamotene | 0.6 | 1431 | 1435 |
| 23 | α-caryophyllene | 1.9 | 1451 | 1452 |
| 24 | allo-aromadendrene | 1.4 | 1455 | 1458 |
| 25 | germacrene D | 0.4 | 1477 | 1484 |
| 26 | β-selinene | 1.1 | 1484 | 1489 |
| 27 | viridiflorene | 0.4 | 1489 | 1496 |
| 27 | bicyclogermacrene | 2.9 | 1490 | 1500 |
| 28 | germacrene A | 1.0 | 1500 | 1508 |
| 29 | germacrene B | 1.6 | 1556 | 1559 |
| 30 | spathulenol | 1.5 | 1576 | 1577 |
| 31 | caryophyllene oxide | 1.9 | 1581 | 1582 |
| Total identified | 97.1 |
| Compound a | 70% EtOH Extract mg/g d.e. (mg/g drug) b | MeOH Extract mg/g d.e. (mg/g Drug) b | UV ʎmax (nm) | [M − H]− (m/z) | MS2 (m/z) Base Peak |
|---|---|---|---|---|---|
| Caftaric acid | 0.35 ± 0.01 (0.07 ± 0.00) | 0.57 ± 0.01 (0.03 ± 0.00) | 327 | 311 | 149 |
| Chlorogenic acid | 0.27 ± 0.01 (0.05 ± 0.00) | 0.05 ± 0.01 (0.003 ± 0.000) | 327 | 353 | 191 |
| Rutin | 0.63 ± 0.01 (0.12 ± 0.01) | 0.56 ± 0.01 (0.02 ± 0.00) | 255, 355 | 609 | 301 |
| Rosmarinic acid | 22.3 ± 0.2 (4.33 ± 0.01) | 21.8 ± 0.3 (1.17 ± 0.02) | 327 | 359 | 161 |
| Extracts and Compounds | DPPH IC50 | ABTS IC50 |
|---|---|---|
| O. campechianum 70% EtOH extract | 11.10 ± 1.13 | 8.58 ± 0.53 |
| O. campechianum MeOH extract | 52.15 ± 2.72 | 15.15 ± 0.52 |
| Rosmarinic acid | 4.31 ± 0.34 | 1.93 ± 0.07 |
| Trolox (positive control) | 3.66 ± 0.29 | 2.14 ± 0.25 |
| O. campechianum EO | 7.77 ± 0.07 | 3.18 ± 0.29 |
| Eugenol | 5.64 ± 0.27 | 8.58 ± 0.53 |
| Extracts and Compounds | MIC (μg/mL) Staphylococcus aureus (ATCC 6538) | MIC (μg/mL) Pseudomonas syringae pv. syringae (ATCC 19310) |
|---|---|---|
| O. campechianum 70% EtOH extract | >2000 | >2000 |
| O. campechianum MeOH extract | >2000 | >2000 |
| Rosmarinic acid | >2000 | >2000 |
| Cloramphenicol (positive control) | 10 | 2.5 |
| O. campechianum EO | >2000 | 250 |
| Eugenol | 1000 | 500 |
| FICindex MIC (EO + FLU) | FICindex MFC (EO + FLU) | EO MIC MFC | FLU MIC MFC | |||
|---|---|---|---|---|---|---|
| C. albicans (AIDS6) | 1.031 (1563 − 4) * | 1.031 (1563 − 4) * | 1563 | 1563 | 128 | 128 |
| C. glabrata (FLU 43976) | 0.562 (781 − 8) * | 0.562 (781 − 8) * | 1563 | 1563 | 128 | 128 |
| C. albicans (ATCC 24433) | 1.004 (3135 − 0.5) * | 1.004 (3135 − 0.5) * | 3135 | 3135 | 128 | 128 |
| Extracts | O. Campechianum EO | O. Campechianum 70%EtOH Extract | O. Campechianum MeOH Extract | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TA98 (t/c) * | TA100 (t/c) * | TA98 (t/c)* | TA100 (t/c) * | TA98 (t/c) * | TA100 (t/c) * | ||||||||
| Conc.% (mg/plate) | −S9 | +S9 | −S9 | +S9 | −S9 | +S9 | −S9 | +S9 | −S9 | +S9 | −S9 | +S9 | |
| 5 (0.25 mg/plate) | 0.9 | 0.7 | 1.0 | 0.8 | 0.7 | 1.0 | 0.9 | 0.8 | 0.4 | 0.9 | 1.2 | 0.9 | |
| 10 (0.5 mg/plate) | 0.9 | 0.5 | 1.0 | 0.8 | 0.7 | 1.2 | 0.9 | 1.0 | 0.7 | 0.9 | 1.1 | 0.9 | |
| 20 (1 mg/plate) | 0.9 | 0.6 | 0.9 | 0.7 | 0.7 | 1.0 | 0.9 | 0.9 | 0.5 | 0.7 | 0.9 | 0.9 | |
| 50 (2.5 mg/plate) | 1.1 | 0.3 | 0.5 | 0.2 | 1.0 | 0.5 | 1.1 | 1.0 | 0.3 | 0.8 | 1.0 | 0.9 | |
| 100 (5 mg/plate) | 0.3 | 0.0 | 0.0 | 0.2 | 0.7 | 0.7 | 1.0 | 0.7 | 0.6 | 0.5 | 1.1 | 1.0 | |
| C+ ** | 4.4 | 4.3 | 5.4 | 4.6 | 4.4 | 4.3 | 5.4 | 4.6 | 3.8 | 4.3 | 5.6 | 4.6 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tacchini, M.; Echeverria Guevara, M.P.; Grandini, A.; Maresca, I.; Radice, M.; Angiolella, L.; Guerrini, A. Ocimum campechianum Mill. from Amazonian Ecuador: Chemical Composition and Biological Activities of Extracts and Their Main Constituents (Eugenol and Rosmarinic Acid). Molecules 2021, 26, 84. https://doi.org/10.3390/molecules26010084
Tacchini M, Echeverria Guevara MP, Grandini A, Maresca I, Radice M, Angiolella L, Guerrini A. Ocimum campechianum Mill. from Amazonian Ecuador: Chemical Composition and Biological Activities of Extracts and Their Main Constituents (Eugenol and Rosmarinic Acid). Molecules. 2021; 26(1):84. https://doi.org/10.3390/molecules26010084
Chicago/Turabian StyleTacchini, Massimo, Monica Paulina Echeverria Guevara, Alessandro Grandini, Immacolata Maresca, Matteo Radice, Letizia Angiolella, and Alessandra Guerrini. 2021. "Ocimum campechianum Mill. from Amazonian Ecuador: Chemical Composition and Biological Activities of Extracts and Their Main Constituents (Eugenol and Rosmarinic Acid)" Molecules 26, no. 1: 84. https://doi.org/10.3390/molecules26010084