Recycling Chocolate Aluminum Wrapping Foil as to Create Electrochemical Metal Strip Electrodes
Abstract
:1. Introduction
2. Results and Discussions
2.1. EDX Measurements for S1 and S2 Electrode Surfaces
2.2. CV for S1 and S2 Electrodes in PB Solution (pH 7)
2.3. Effect of CV Scan Rates on the Double-Layer Capacitance (Cdl)
2.4. Chronoamperometric Measurements for S1 and S2 Electrodes
2.5. Electrochemical Impedance Spectroscopy (EIS) for S1 and S2 Electrodes
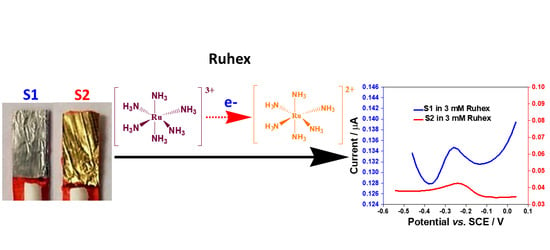
2.6. Electrochemical Responses of S1 and S2 Electrodes toward the Redox Reactions of Ruthenium Hexamine (Ruhex)
3. Material and Methods
3.1. Chemicals
3.2. Fabrication of the Electrode Strips
3.3. Electrochemical Characterizations of the Electrode Strips
3.4. Data Analysis
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Honeychurch, K.C.; Rymansaib, Z.; Iravani, P. Anodic stripping voltammetric determination of zinc at a 3-D printed carbon nanofiber–graphite–polystyrene electrode using a carbon pseudo-reference electrode. Sens. Actuators B Chem. 2018, 267, 476–482. [Google Scholar] [CrossRef]
- Varela-Guerrero, V.; Yañez, G.M.; Barrera, G.R.; Morales, M.A. García-Morales. Use of recycled aluminum—Polyethylene composite films as anodic electrodes for electrocoagulation of wastewater. Int. J. Electrochem. Sci. 2006, 9, 1034–1043. [Google Scholar]
- Kim, H.S.; Shin, E.J. Re-synthesis and electrochemical characteristics of LiFePO4 cathode materials recycled from scrap electrodes. Bull. Korean Chem. Soc. 2013, 34, 851–855. [Google Scholar] [CrossRef] [Green Version]
- Moro, G.; Bottari, F.; Van Loon, J.; Du Bois, E.; De Wael, K.; Moretto, L.M. Disposable electrodes from waste materials and renewable sources for (bio)electroanalytical applications. Biosens. Bioelectron. 2019, 146, 111758. [Google Scholar] [CrossRef]
- Singh, A.A.; Afrin, S.; Karim, Z. Green Composites: Versatile Material for Future; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Ramli, J.; Jeefferie, A.R.; Mahat, M.M. Effects of UV curing exposure time to the mechanical and physical properties of the epoxy and vinyl ester fiber glass laminates composites. ARPN J. Eng. Appl. Sci. 2011, 6, 104–109. [Google Scholar]
- Torres, F.; Rodriguez, S.; Saavedra, A.C. Green composite materials from biopolymers reinforced with agroforestry waste. J. Polym. Environ. 2019, 27, 2651–2673. [Google Scholar] [CrossRef]
- Pradela-Filho, L.A.; Andreotti, I.A.; Carvalho, J.H.; Araújo, D.A.; Orzari, L.O.; Gatti, A.; Takeuchi, R.M.; Santos, A.L.; Janegitz, B.C. Glass varnish-based carbon conductive ink: A new way to produce disposable electrochemical sensors. Sens. Actuators B Chem. 2020, 305, 127433. [Google Scholar] [CrossRef]
- Andreotti, I.A.D.A.; Orzari, L.O.; Camargo, J.R.; Faria, R.C.; Marcolino-Junior, L.H.; Bergamini, M.F.; Gatti, A.; Janegitz, B.C. Disposable and flexible electrochemical sensor made by recyclable material and low cost conductive ink. J. Electroanal. Chem. 2019, 840, 109–116. [Google Scholar] [CrossRef]
- Rodic, L.; Wilson, D.C. Resolving governance issues to achieve priority sustainable development goals related to solid waste management in developing countries. Sustainability 2017, 9, 404. [Google Scholar] [CrossRef] [Green Version]
- González-Sánchez, M.-I.; Gómez-Monedero, B.; Agrisuelas, J.; Valero, E. Recycling metals from spent screen-printed electrodes while learning the fundamentals of electrochemical sensing. J. Chem. Educ. 2018, 95, 847–851. [Google Scholar] [CrossRef]
- De Oliveira, G.C.M.; Camargo, J.R.; Vieira, N.C.S.; Janegitz, B.C. A new disposable electrochemical sensor on medical adhesive tape. J. Solid State Electrochem. 2020, 24, 2271–2278. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Qiu, M.; Yulong, C.; Zhang, X.; Sun, X.W.; Chen, R. Preparation of aluminum nanomesh thin films from an anodic aluminum oxide template as transparent conductive electrodes. Sci. Rep. 2016, 6, 20114. [Google Scholar] [CrossRef] [Green Version]
- Farehanim, M.A.; Hashim, U.; Soin, N.; Azman, A.; Norhafizah, S.; Fatin, M.; Ayub, R. Fabrication of aluminum interdigitated electrode for biosensor application using conventional lithography. Adv. Mater. Res. 2015, 1109, 118–122. [Google Scholar] [CrossRef]
- Jones, C.E. Packaging. In Industrial Chocolate Manufacture and Use, 5th ed.; Willey-Blackwell: Hoboken, NJ, USA, 2017; pp. 620–653. [Google Scholar]
- Kalhapure, M.; Dighe, P. Impact of silicon content on mechanical properties of aluminum alloys. Int. J. Sci. Res. 2013, 14, 38–40. [Google Scholar]
- Bučko, M.; Gemeiner, P.; Vikartovska, A.; Mislovičová, D.; Lacík, I.; Tkac, J. Coencapsulation of oxygen carriers and glucose oxidase in polyelectrolyte complex capsules for the enhancement of D-gluconic acid and δ-gluconolactone production. Artif. Cells Blood Substit. Biotechnol. 2010, 38, 90–98. [Google Scholar] [CrossRef]
- Lee, H.; Xu, F.; Jeffcoate, C.S.; Isaacs, H.S. Cyclic Polarization behavior of aluminum oxide films in near neutral solutions. Electrochem. Solid State Lett. 2001, 4, B31. [Google Scholar] [CrossRef]
- Diggle, J.W.; Downie, T.C.; Goulding, C.W. Anodic oxide films on aluminum. Chem. Rev. 1969, 69, 365–405. [Google Scholar] [CrossRef]
- Pryor, M.J. Electrode reactions on oxide covered aluminum. Z. für Elektrochem. Ber. der Bunsenges. für Phys. Chem. 1958, 62, 782–794. [Google Scholar]
- Lee, J.S.M.; Briggs, M.E.; Hu, C.C.; Cooper, A.I. Controlling electric double-layer capacitance and pseudocapacitance in heteroatom-doped carbons derived from hypercrosslinked microporous polymers. Nano Energy 2018, 46, 277–289. [Google Scholar] [CrossRef]
- Ahmed, M.M.M.; Imae, T. Graphene-based nanolayers toward energy storage device. In Nanolayer Research; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Ge, Y.; Xie, X.; Roscher, J.; Holze, R.; Qu, Q. How to measure and report the capacity of electrochemical double layers, supercapacitors, and their electrode materials. J. Solid State Electrochem. 2020, 24, 1–16. [Google Scholar] [CrossRef]
- Wang, J. Fundamental concepts. In Analytical Chemistry, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2000; pp. 1–27. [Google Scholar]
- Moretto, L.; Pepe, N.; Ugo, P. Voltammetry of redox analytes at trace concentrations with nanoelectrode ensembles. Talanta 2004, 62, 1055–1060. [Google Scholar] [CrossRef]
- Ślesiński, A.; Frackowiak, E. Determination of accurate electrode contribution during voltammetry scan of electrochemical capacitors. J. Solid State Electrochem. 2018, 22, 2135–2139. [Google Scholar] [CrossRef] [Green Version]
- Forster, R.J. Reversibly adsorbed monolayers on microelectrodes: Effect of potential on the adsorption thermodynamics. Anal. Chem. 1996, 68, 3143–3150. [Google Scholar] [CrossRef]
- Moulton, S.E.; Barisci, J.N.; Bath, A.; Stella, R.; Wallace, G.G. Investigation of Ig.G adsorption and the effect on electrochemical responses at titanium dioxide electrode. Langmuir 2005, 21, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Pyun, S.I.; Shin, H.C. Kinetics of double-layer charging/discharging of activated carbon electrodes: Role of surface acidic functional groups. J. Electrochem. Soc. 2002, 149, A93–A98. [Google Scholar] [CrossRef]
- Hamzah, H.H.B.; Keattch, O.; Covill, D.; Patel, B.A. The effects of printing orientation on the electrochemical behaviour of 3D printed acrylonitrile butadiene styrene (ABS)/carbon black electrodes. Sci. Rep. 2018, 8, 9135. [Google Scholar] [CrossRef] [Green Version]
- White, K.A.; Mulberry, G.; Kim, B. Parallel 1024-ch cyclic voltammetry on monolithic CMOS electrochemical detector array. IEEE Sens. J. 2020, 20, 4395–4402. [Google Scholar] [CrossRef]










| Electrode | Parameter | Electrode Number | Mean ± S.D | |||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| S1 | Areal (As/Fv) | 0.48 | 0.37 | 0.50 | 0.59 | 0.49 ± 0.090 |
| ρ | 0.96 | 0.74 | 1.00 | 1.18 | 0.97 ± 0.18 | |
| S2 | Areal (As/Fv) | 0.64 | 0.51 | 0.68 | 0.72 | 0.64 ± 0.091 |
| ρ | 1.28 | 1.02 | 1.36 | 1.44 | 1.28 ± 0.18 | |
| Parameter | GC | S1 | S2 | |||
|---|---|---|---|---|---|---|
| Value | Error/% | Value | Error/% | Value | Error/% | |
| Solution resistance (Rs) | 277.32 Ω | 0.58 | 74.54 Ω | 5.31 | 104.42 Ω | 8.21 |
| Double layer capacitance (Cdl) | 1.42 μF | 2.56 | 0.30 μF | 6.69 | 0.41 μF | 17.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamzah, H.H.; Saleh, N.H.; Patel, B.A.; Mahat, M.M.; Shafiee, S.A.; Sönmez, T. Recycling Chocolate Aluminum Wrapping Foil as to Create Electrochemical Metal Strip Electrodes. Molecules 2021, 26, 21. https://doi.org/10.3390/molecules26010021
Hamzah HH, Saleh NH, Patel BA, Mahat MM, Shafiee SA, Sönmez T. Recycling Chocolate Aluminum Wrapping Foil as to Create Electrochemical Metal Strip Electrodes. Molecules. 2021; 26(1):21. https://doi.org/10.3390/molecules26010021
Chicago/Turabian StyleHamzah, Hairul Hisham, Nur Hidayah Saleh, Bhavik Anil Patel, Mohd Muzamir Mahat, Saiful Arifin Shafiee, and Turgut Sönmez. 2021. "Recycling Chocolate Aluminum Wrapping Foil as to Create Electrochemical Metal Strip Electrodes" Molecules 26, no. 1: 21. https://doi.org/10.3390/molecules26010021