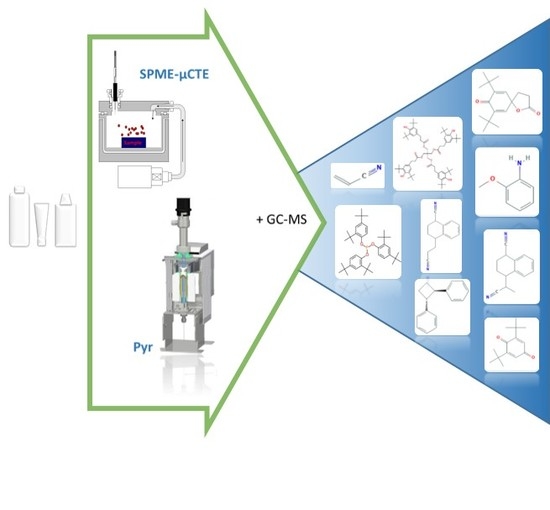
Identification of Potential Extractables and Leachables in Cosmetic Plastic Packaging by Microchambers-Thermal Extraction and Pyrolysis-Gas Chromatography-Mass Spectrometry
Abstract
:1. Introduction
2. Results
2.1. Identification of Released Compounds
2.2. Comparison with Simulants Study
2.3. Toxicological Aspects
3. Materials and Methods
3.1. Samples
3.2. Samples Preparation
3.3. Instrumentation and Conditions
3.4. Qualitative Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thompson, R.C.; Moore, C.J.; Saal, F.S.V.; Swan, S. Plastics, the environment and human health: Current consensus and future trends. Philos. Trans. R. Soc. B: Biol. Sci. 2009, 364, 2153–2166. [Google Scholar] [CrossRef] [PubMed]
- Bradley, E.; Coulier, L. An Investigation into the Reaction and Breakdown Products from Starting Substances Used to Produce Food Contact Plastics; Food Standards Agency: London, UK, 2007.
- Cao, X.-L. Determination of phthalates and adipate in bottled water by headspace solid-phase microextraction and gas chromatography/mass spectrometry. J. Chromatogr. A 2008, 1178, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Pan, X.; Yuan, S.; Wang, Q. Plasticizer Contamination in Edible Vegetable Oil in a U.S. Retail Market. J. Agric. Food Chem. 2013, 61, 9502–9509. [Google Scholar] [CrossRef] [PubMed]
- Lau, O.-W.; Wong, S.-K. Contamination in food from packaging material. J. Chromatogr. A 2000, 882, 255–270. [Google Scholar] [CrossRef]
- Fasano, E.; Bono-Blay, F.; Cirillo, T.; Montuori, P.; Lacorte, S. Migration of phthalates, alkylphenols, bisphenol A and di(2-ethylhexyl)adipate from food packaging. Food Control. 2012, 27, 132–138. [Google Scholar] [CrossRef] [Green Version]
- Gimeno, P.; Maggio, A.-F.; Bousquet, C.; Quoirez, A.; Civade, C.; Bonnet, P.-A. Analytical method for the identification and assay of 12 phthalates in cosmetic products: Application of the ISO 12787 international standard “Cosmetics–Analytical methods–Validation criteria for analytical results using chromatographic techniques”. J. Chromatogr. A 2012, 1253, 144–153. [Google Scholar] [CrossRef]
- Bignardi, C.; Cavazza, A.; Laganà, C.; Salvadeo, P.; Corradini, C. Release of non-intentionally added substances (NIAS) from food contact polycarbonate: Effect of ageing. Food Control. 2017, 71, 329–335. [Google Scholar] [CrossRef]
- Muncke, J. Endocrine disrupting chemicals and other substances of concern in food contact materials: An updated review of exposure, effect and risk assessment. J. Steroid Biochem. Mol. Biol. 2011, 127, 118–127. [Google Scholar] [CrossRef]
- Nerín, C.; Alfaro, P.; Aznar, M.; Domeño, C. The challenge of identifying non-intentionally added substances from food packaging materials: A review. Anal. Chim. Acta 2013, 775, 14–24. [Google Scholar] [CrossRef]
- Amiridou, D.; Voutsa, D. Alkylphenols and phthalates in bottled waters. J. Hazard. Mater. 2011, 185, 281–286. [Google Scholar] [CrossRef]
- Fierens, T.; Servaes, K.; Van Holderbeke, M.; Geerts, L.; De Henauw, S.; Sioen, I.; Vanermen, G. Analysis of phthalates in food products and packaging materials sold on the Belgian market. Food Chem. Toxicol. 2012, 50, 2575–2583. [Google Scholar] [CrossRef] [PubMed]
- Cacho, J.; Campillo, N.; Viñas, P.; Hernández-Córdoba, M. Determination of alkylphenols and phthalate esters in vegetables and migration studies from their packages by means of stir bar sorptive extraction coupled to gas chromatography–mass spectrometry. J. Chromatogr. A 2012, 1241, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Casajuana, N.; Lacorte, S. New Methodology for the Determination of Phthalate Esters, Bisphenol A, Bisphenol A Diglycidyl Ether, and Nonylphenol in Commercial Whole Milk Samples. J. Agric. Food Chem. 2004, 52, 3702–3707. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, Z.; Liu, L.; Li, Y.; Ren, N.; Kannan, K. Occurrence and Profiles of Phthalates in Foodstuffs from China and Their Implications for Human Exposure. J. Agric. Food Chem. 2012, 60, 6913–6919. [Google Scholar] [CrossRef]
- Gimeno, P.; Thomas, S.; Bousquet, C.; Maggio, A.-F.; Civade, C.; Brenier, C.; Bonnet, P.-A. Identification and quantification of 14 phthalates and 5 non-phthalate plasticizers in PVC medical devices by GC–MS. J. Chromatogr. B 2014, 949, 99–108. [Google Scholar] [CrossRef]
- Jenke, D.R.; Castner, J.; Egert, T.; Feinberg, T.; Hendricker, A.; Houston, C.; Hunt, D.G.; Lynch, M.; Shaw, A.; Nicholas, K.; et al. Extractables Characterization for Five Materials of Construction Representative of Packaging Systems Used for Parenteral and Ophthalmic Drug Products. PDA J. Pharm. Sci. Technol. 2013, 67, 448–511. [Google Scholar] [CrossRef]
- Roberts, D.; Feilden, A.; Barlow, R.; D’Silva, K.; Silcock, P. Confident Identification of Leachable Impurities from Pharmaceutical Container Closure Materials Using Orbitrap-Mass-Spectrometer-Based GC-MS; Application Note; Thermo Scientific: Waltham, MA, USA, 2016. [Google Scholar]
- Pan, C.; Harmon, F.; Toscano, K.; Liu, F.; Vivilecchia, R. Strategy for identification of leachables in packaged pharmaceutical liquid formulations. J. Pharm. Biomed. Anal. 2008, 46, 520–527. [Google Scholar] [CrossRef]
- Bourdeaux, D.; Yessaad, M.; Chennell, P.; Larbre, V.; Eljezi, T.; Bernard, L.; Sautou, V. Analysis of PVC plasticizers in medical devices and infused solutions by GC–MS. J. Pharm. Biomed. Anal. 2016, 118, 206–213. [Google Scholar] [CrossRef]
- Thakare, V.; Mayr, B.; Artenjak, A.; Nianios, D.; Sest, M.; Müller, M.; Maltsev, O.V.; Nowicki, K.; Mischo, A.; Ehrenstrasser, C.; et al. Investigation of drug product and container-closure interactions: A case study of diluent containing prefilled syringe. Eur. J. Pharm. Biopharm. 2019, 140, 67–77. [Google Scholar] [CrossRef]
- Thomas, C.; Siong, D.; Pirnay, S. Evaluation of the content - containing interaction in cosmetic products using gas chromatography-mass spectrometry. Int. J. Cosmet. Sci. 2014, 36, 327–335. [Google Scholar] [CrossRef]
- Shen, H.-Y.; Jiang, H.-L.; Mao, H.-L.; Pan, G.; Zhou, L.; Cao, Y.-F. Simultaneous determination of seven phthalates and four parabens in cosmetic products using HPLC-DAD and GC-MS methods. J. Sep. Sci. 2007, 30, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, C.; Wang, X.; Hao, N.; Liu, J. Determination of phthalate esters in cosmetics by gas chromatography with flame ionization detection and mass spectrometric detection. Int. J. Cosmet. Sci. 2005, 27, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Kannan, K. A Survey of Phthalates and Parabens in Personal Care Products from the United States and Its Implications for Human Exposure. Environ. Sci. Technol. 2013, 47, 14442–14449. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, T.; Latini, G.; Castaldi, M.A.; DiPaola, L.; Fasano, E.; Esposito, F.; Scognamiglio, G.; Di Francesco, F.; Cobellis, L. Exposure to Di-2-Ethylhexyl Phthalate, Di-N-Butyl Phthalate and Bisphenol A through Infant Formulas. J. Agric. Food Chem. 2015, 63, 3303–3310. [Google Scholar] [CrossRef]
- Feng, C.-H.; Jiang, S.-R. Micro-scale quantitation of ten phthalate esters in water samples and cosmetics using capillary liquid chromatography coupled to UV detection: Effective strategies to reduce the production of organic waste. Microchim. Acta 2012, 177, 167–175. [Google Scholar] [CrossRef]
- Viñas, P.; Campillo, N.; Pastor-Belda, M.; Oller, A.; Hernández-Córdoba, M. Determination of phthalate esters in cleaning and personal care products by dispersive liquid–liquid microextraction and liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2015, 1376, 18–25. [Google Scholar] [CrossRef]
- Ferrer, E.; Santoni, E.; Vittori, S.; Font, G.; Mañes, J.; Sagratini, G. Simultaneous determination of bisphenol A, octylphenol, and nonylphenol by pressurised liquid extraction and liquid chromatography–tandem mass spectrometry in powdered milk and infant formulas. Food Chem. 2011, 126, 360–367. [Google Scholar] [CrossRef]
- Lateef, S.S. Extractables and Leachables Detected in Ophthalmic Drug Products—Detection and Identification Using High-Resolution LC/MS/MS.; Agilent Technologies Inc.: Santa Clara, CA, USA, 2016. [Google Scholar]
- Zhang, Y.; Sun, S.; Xing, X.; Du, Z.; Guo, Q.; Yu, W. Detection and Identification of Leachables in Vaccine from Plastic Packaging Materials Using UPLC-QTOF MS with Self-Built Polymer Additives Library. Anal. Chem. 2016, 88, 6749–6757. [Google Scholar] [CrossRef]
- Pinguet, J.; Kerckhove, N.; Eljezi, T.; Lambert, C.; Moreau, E.; Bernard, L.; Boeuf, B.; Decaudin, B.; Genay, S.; Masse, M.; et al. New SPE-LC-MS/MS method for the simultaneous determination in urine of 22 metabolites of DEHP and alternative plasticizers from PVC medical devices. Talanta 2019, 198, 377–389. [Google Scholar] [CrossRef]
- Bernard, L.; Cueff, R.; Breysse, C.; Décaudin, B.; Sautou, V. Migrability of PVC plasticizers from medical devices into a simulant of infused solutions. Int. J. Pharm. 2015, 485, 341–347. [Google Scholar] [CrossRef]
- Ubeda, S.; Aznar, M.; Rosenmai, A.K.; Vinggaard, A.M.; Nerín, C. Migration studies and toxicity evaluation of cyclic polyesters oligomers from food packaging adhesives. Food Chem. 2020, 311, 125918. [Google Scholar] [CrossRef] [PubMed]
- Murat, P.; Ferret, P.-J.; Coslédan, S.; Simon, V. Assessment of targeted non-intentionally added substances in cosmetics in contact with plastic packagings. Analytical and toxicological aspects. Food Chem. Toxicol. 2019, 128, 106–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murat, P.; Ferret, P.-J.; Coslédan, S.; Simon, V. Development of a HS-SPME-GC-MS method for the analysis of phthalates in glycerin and liquid paraffin: Application to safety evaluation of cosmetic packagings. Anal. Bioanal. Chem. 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- European Commission Commission Regulation (EU). n°10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to Come into Contact with Food; European Union: Brussels, Belgium, 2011. [Google Scholar]
- Takahashi, F.; Kobayashi, M.; Kobayashi, A.; Kobayashi, K.; Asamura, H. High-Frequency Heating Extraction Method for Sensitive Drug Analysis in Human Nails. Molecules 2018, 23, 3231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, Y.; Koga, N. Thermal Decomposition of Maya Blue: Extraction of Indigo Thermal Decomposition Steps from a Multistep Heterogeneous Reaction Using a Kinetic Deconvolution Analysis. Molecules 2019, 24, 2515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, T.; Bertron, A.; Escadeillas, G.; Ringot, E.; Simon, V. BTEX abatement by photocatalytic TiO2-bearing coatings applied to cement mortars. Build. Environ. 2014, 71, 186–192. [Google Scholar] [CrossRef]
- Simon, V.; Uitterhaegen, E.; Robillard, A.; Ballas, S.; Véronèse, T.; Vilarem, G.; Merah, O.; Talou, T.; Evon, P. VOC and carbonyl compound emissions of a fiberboard resulting from a coriander biorefinery: Comparison with two commercial wood-based building materials. Environ. Sci. Pollut. Res. 2020, 1–13. [Google Scholar] [CrossRef]
- Candelier, K.; Dumarcay, S.; Pétrissans, A.; Pétrissans, M.; Kamdem, P.; Gérardin, P. Thermodesorption coupled to GC–MS to characterize volatiles formation kinetic during wood thermodegradation. J. Anal. Appl. Pyrolysis 2013, 101, 96–102. [Google Scholar] [CrossRef]
- Nohr, M.; Horn, W.; Wiegner, K.; Richter, M.; Lorenz, W. Development of a material with reproducible emission of selected volatile organic compounds – μ-Chamber study. Chemosphere 2014, 107, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Groh, K.; Backhaus, T.; Almroth, B.C.; Geueke, B.; Inostroza, P.; Lennquist, A.; Leslie, H.A.; Maffini, M.; Slunge, D.; Trasande, L.; et al. Overview of known plastic packaging-associated chemicals and their hazards. Sci. Total Environ. 2019, 651, 3253–3268. [Google Scholar] [CrossRef] [PubMed]
- Jenke, D. Compatibility of Pharmaceutical Products and Contact Materials: Safety Considerations Associated with Extractables and Leachables; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2009; ISBN 978-1-118-67947-0. [Google Scholar]
- Burman, L.; Albertsson, A.-C.; Höglund, A. Solid-phase microextraction for qualitative and quantitative determination of migrated degradation products of antioxidants in an organic aqueous solution. J. Chromatogr. A 2005, 1080, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Alin, J.; Hakkarainen, M. Microwave Heating Causes Rapid Degradation of Antioxidants in Polypropylene Packaging, Leading to Greatly Increased Specific Migration to Food Simulants As Shown by ESI-MS and GC-MS. J. Agric. Food Chem. 2011, 59, 5418–5427. [Google Scholar] [CrossRef] [PubMed]
- Alin, J.; Hakkarainen, M. Combined Chromatographic and Mass Spectrometric Toolbox for Fingerprinting Migration from PET Tray during Microwave Heating. J. Agric. Food Chem. 2013, 61, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Dutra, C.; Pezo, D.; Freire, M.T.D.A.; Nerin, C.; Reyes, F. Determination of volatile organic compounds in recycled polyethylene terephthalate and high-density polyethylene by headspace solid phase microextraction gas chromatography mass spectrometry to evaluate the efficiency of recycling processes. J. Chromatogr. A 2011, 1218, 1319–1330. [Google Scholar] [CrossRef]
- Ibarra, V.A.G.; Sendón, R.; Bustos, J.; Losada, P.P.; De Quirós, A.R.-B. Estimates of dietary exposure of Spanish population to packaging contaminants from cereal based foods contained in plastic materials. Food Chem. Toxicol. 2019, 128, 180–192. [Google Scholar] [CrossRef]
- Löschner, D.; Rapp, T.; Schlosser, F.-U.; Schuster, R.; Stottmeister, E.; Zander, S. Experience with the application of the draft European Standard prEN 15768 to the identification of leachable organic substances from materials in contact with drinking water by GC-MS. Anal. Methods 2011, 3, 2547. [Google Scholar] [CrossRef]
- Richardson, S.D.; Collette, T.; Price, P.C.; Genicola, F.A.; Jenks, J.W.; Thruston, A.D.; Ellington, J.J. Identification of drinking water contaminants in the course of a childhood cancer investigation in Toms River, New Jersey. J. Expo. Sci. Environ. Epidemiol. 1999, 9, 200–216. [Google Scholar] [CrossRef] [Green Version]
- O’Neil, M.J. (Ed.) The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 14th ed; Merck Handbooks; Merck: Whitehouse Station, NJ, USA, 2006; ISBN 978-0-911910-00-1. [Google Scholar]
- Biryol, D.; Nicolas, C.I.; Wambaugh, J.F.; Phillips, K.; Isaacs, K.K. High-throughput dietary exposure predictions for chemical migrants from food contact substances for use in chemical prioritization. Environ. Int. 2017, 108, 185–194. [Google Scholar] [CrossRef]
- COWI. Danish Technological Institute Hazardous Substances in Plastic Materials; Norwegian Climate and Pollution Agency: Oslo, Norway, 2013. [Google Scholar]
- Stenmarck, Å.; Belleza, E.L.; Fråne, A.; Busch, N.; Larsen, Å.; Wahlström, M. Hazardous Substances in Plastics:-Ways to Increase Recycling; Nordisk Ministerråd: Copenhagen, Denmark, 2017. [Google Scholar]
- Su, Q.Z.; Vera, P.; Van de Wiele, C.; Nerín, C.; Lin, Q.B.; Zhong, H.N. Non-target screening of (semi-)volatiles in food-grade polymers by comparison of atmospheric pressure gas chromatography quadrupole time-of-flight and electron ionization mass spectrometry. Talanta 2019, 202, 285–296. [Google Scholar] [CrossRef]
- Rajbux, C.; Pereira, J.; Selbourne, M.D.C.; Costa-Pinto, A.R.; Poças, F. Assessment of baby Bibs. GC-MS screening, migration into saliva and insight of toxicity with QSAR tools. Food Control. 2020, 109, 106951. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency CPCat: Chemical and Product Categories. Available online: https://actor.epa.gov/cpcat/faces/search.xhtml;jsessionid=6A32B2AEE39BDEF95E8F4E39E7698394 (accessed on 24 February 2020).
- Commission Européenne CosIng. Available online: https://ec.europa.eu/growth/tools-databases/cosing/ (accessed on 18 February 2020).
- Ibarra, V.A.G.; De Quirós, A.R.-B.; Losada, P.P.; Sendón, R. Non-target analysis of intentionally and non intentionally added substances from plastic packaging materials and their migration into food simulants. Food Packag. Shelf Life 2019, 21, 100325. [Google Scholar] [CrossRef]
- Wypych, A. Databook of Plasticizers; Elsevier: Scarborough, UK, 2017; ISBN 978-1-927885-15-4. [Google Scholar]
- Flick, E.W. Plastics Design Library Plastics Additives Database 2004; William Andrew: Norwich, NY, USA, 2004. [Google Scholar]
- Bolgar, M.; Hubball, J.; Groeger, J.; Meronek, S. (Eds.) Handbook for the Chemical Analysis of Plastic and Polymer Additives, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-1-4398-6075-5. [Google Scholar]
Sample Availability: Not Available. |





| Compound | Molecular Formula | CAS# | Function | Cramer Class | P1 | P2 | P3 | P4 | P5 | P6 | P7 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acrylonitrile | C3H3N | 107-13-1 | Intermediate in the synthesis of antioxidants and dyes, monomer [53,54] | III | Pyr | ||||||
| 2-methoxy-benzeneamine | C7H9NO | 90-04-0 | Used for dyes manufacturing, printing ink [55] | III | Pyr | ||||||
| Styrene | C8H8 | 100-42-5 | Monomer, intermediate [54,56] | I | Pyr | ||||||
| n-butyl-methacrylate | C8H14O2 | 97-88-1 | Monomer, additive [54] | I | Pyr | ||||||
| Alpha-methylstyrene | C9H10 | 98-83-9 | Monomer, additive [54] | I | Pyr | ||||||
| 4-propyl-benzaldehyde | C10H12O | 28785-06-0 | Additive degradation product [57] | I | Pyr | ||||||
| Diethyl phthalate (DEP) | C12H14O4 | 84-66-2 | Solvent, plasticizer, extractable associated with polyethylene and PET [45,58] | I | TD | TD | TD | TD | |||
| Linalyl acetate | C12H20O2 | 115-95-7 | Used for plastics manufacturing, lubricant, and additives [59] | I | Pyr | ||||||
| 1-dodecanol | C12H26O | 112-53-8 | Plasticizer, lubricant [59] | I | TD | ||||||
| 1-Hydroxycyclohexyl phenyl ketone | C13H16O2 | 947-19-3 | Photo-initiator [58] | I | TD | TD | TD | TD | TD | ||
| Diisobutyl glutarate | C13H24O4 | 71195-64-7 | Plasticizer [60] | I | TD | ||||||
| 4-(1-Cyanoethyl)-1,2,3,4-tetrahydronaphthalene-1-carbonitrile | C14H14N2 | 57964-39-3 | By-product of SAN production process [52] | III | PyrTD | ||||||
| 4-Cyano-1,2,3,4-tetrahydro-1-naphthaleneacetonitrile | C14H14N2 | 57964-40-6 | By-product of SAN production process [52] | III | PyrTD | ||||||
| 2,6-di-tert-butyl-P-benzoquinone | C14H20O2 | 719-22-2 | Degradation product, extractable associated with polyethylene materials [45,61] | III | TD | ||||||
| 2,4-di-tert-butylphenol (Dtbp) | C14H22O | 96-76-4 | UV stabilizer, antioxidant, degradation product [58,61] | I | Pyr | PyrTD | PyrTD | PyrTD | PyrTD | PyrTD | |
| Isodecyl methacrylate | C14H26O2 | 29964-84-9 | Monomer [54] | II | Pyr | ||||||
| 1-tetradecene | C14H28 | 1120-36-1 | Monomer, additive [54] | I | TD | ||||||
| 2-ethylhexyl benzoate | C15H22O2 | 5444-75-7 | Plasticizer [62] | I | TD | ||||||
| Isopropyl dodecanoate | C15H30O2 | 10233-13-3 | Additive [54] | I | TD | TD | TD | TD | |||
| Trans-1,2-Diphenylcyclobutane | C16H16 | 20071-09-4 | Extractable associated with polystyrene materials [45] | III | Pyr | ||||||
| Di-n-butyl phthalate (DnBP) | C16H22O4 | 84-74-2 | Plasticizer, catalyst, extractable associated with polyethylene, PET, and polystyrene materials [45,56,58] | I | TD | TD | |||||
| Diisobutyl phthalate (DiBP) | C16H22O4 | 84-69-5 | Plasticizer, present in printing ink, extractable associated with polyethylene materials [45,58,61] | I | TD | TD | TD | ||||
| 2,2,4-trimethyl-1,3-pentanediol diisobutyrate | C16H30O4 | 6846-50-0 | Plasticizer, monomer [54,58] | I | TD | TD | TD | ||||
| n-hexadecanoic acid | C16H32O2 | 57-10-3 | Slip agent degradant, monomer, extractable associated with polyethylene and PET [45,54,61] | I | TD | TD | |||||
| 1-decanol-2 hexyl | C16H34O | 2425-77-6 | Additive [54] | I | TD | ||||||
| 1-hexadecanol | C16H34O | 36653-82-4 | Monomer, additive [54] | I | TD | ||||||
| 7,9-di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione (DtbO) | C17H24O3 | 82304-66-3 | Degradation product, impurity of Irganox 1076 [58,61] | III | PyrTD | PyrTD | TD | PyrTD | TD | TD | TD |
| Isopropyl myristate | C17H34O2 | 110-27-0 | Plasticizer, lubricant, [61] | I | TD | TD | TD | TD | |||
| Methyl palmitate | C17H34O2 | 112-39-0 | Intermediate for resins and defoamer in food contact coatings [61] | I | Pyr | ||||||
| 1-octadecanol | C18H38O | 112-92-5 | Ink solvent, plasticizer [61] | I | TD | ||||||
| Butyl 2-ethylhexyl phthalate | C20H30O4 | 85-69-8 | Plasticizer [63] | I | TD | ||||||
| Butyl octyl phthalate | C20H30O4 | 84-78-6 | Plasticizer [63] | I | TD | ||||||
| Bis(2-ethylhexyl) adipate | C22H42O4 | 103-23-1 | Plasticizer, extractable associated with PET [45,61] | I | TD | ||||||
| Bis(2-ethylhexyl) phthalate (DEHP) | C24H38O4 | 117-81-7 | Plasticizer, extractable associated with PET and polystyrene materials [45,58] | I | TD | ||||||
| Diisooctyl phthalate | C24H38O4 | 27554-26-3 | Plasticizer [64] | I | TD |
| Code | Material | Appearance | Shape and Type |
|---|---|---|---|
| P1 | 100% PP | Opaque, white | Elliptical bottle |
| P2 | 100% PP | Opaque, green | Cylindrical bottle |
| P3 | 100% SAN | Opaque, white | Cylindrical bottle |
| P4 | 100% HDPE | Opaque, white | Cylindrical bottle |
| P5 | 70% LLDPE/30% XLDPE | Opaque, white | Cylindrical tube |
| P6 | COEX 70% LLDPE/30% XLDPE//EVOH | Opaque, white | Cylindrical tube |
| P7 | 70% HDPE/30% LLDPE | Opaque, white | Cylindrical tube |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murat, P.; Harohalli Puttaswamy, S.; Ferret, P.-J.; Coslédan, S.; Simon, V. Identification of Potential Extractables and Leachables in Cosmetic Plastic Packaging by Microchambers-Thermal Extraction and Pyrolysis-Gas Chromatography-Mass Spectrometry. Molecules 2020, 25, 2115. https://doi.org/10.3390/molecules25092115
Murat P, Harohalli Puttaswamy S, Ferret P-J, Coslédan S, Simon V. Identification of Potential Extractables and Leachables in Cosmetic Plastic Packaging by Microchambers-Thermal Extraction and Pyrolysis-Gas Chromatography-Mass Spectrometry. Molecules. 2020; 25(9):2115. https://doi.org/10.3390/molecules25092115
Chicago/Turabian StyleMurat, Pauline, Sowmya Harohalli Puttaswamy, Pierre-Jacques Ferret, Sylvie Coslédan, and Valérie Simon. 2020. "Identification of Potential Extractables and Leachables in Cosmetic Plastic Packaging by Microchambers-Thermal Extraction and Pyrolysis-Gas Chromatography-Mass Spectrometry" Molecules 25, no. 9: 2115. https://doi.org/10.3390/molecules25092115