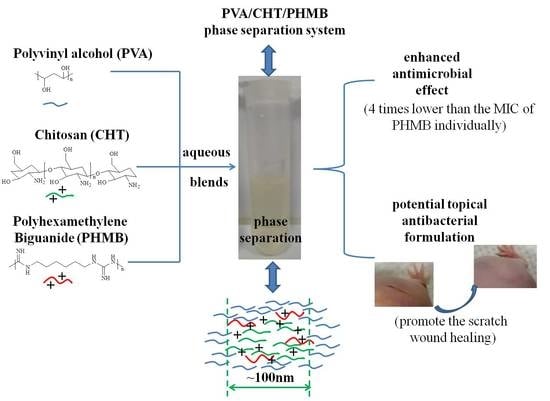
Polyvinyl Alcohol/Chitosan/Polyhexamethylene Biguanide Phase Separation System: A Potential Topical Antibacterial Formulation with Enhanced Antimicrobial Effect
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PVA/CHT/PHMB Blends
2.3. Bacterial Strains and Culture Conditions
2.4. Assays for Antibacterial Activity
2.5. DLS and Zeta Potential Measurement of PVA/CHT/PHMB Blends
2.6. Rheological Measurement of PVA/CHT/PHMB Blends
2.7. Preparation of PVA/CHT/PHMB Blends Formed Film
2.8. Observation of the Surface Morphology of PVA/CHT/PHMB Blends Formed Film
2.9. WVTR (Water Vapor Transmission Rate) of PVA/CHT/PHMB Blends Formed Film
2.10. Mechanical Properties of PVA/CHT/PHMB Blends Formed Film
2.11. Scratch Wound Healing Test
3. Results and Discussion
3.1. The Preparation of Blends
3.2. The Antibacterial Activity of Blends
3.3. PVA/CHT/PHMB Blends Film Forming Characterization
3.4. WVTR (Water Vapor Transmission Rate) of PVA/CHT/PHMB Blends Formed Film
3.5. Mechanical Properties of PVA/CHT/PHMB Blends Formed Film
3.6. Scratch Wound Healing Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lio, P.A.; Kaye, E.T. Topical antibacterial agents. Infect. Dis. Clin. N. Am. 2009, 23, 945–963. [Google Scholar] [CrossRef]
- Spann, C.T.; Tutrone, W.D.; Weinberg, J.M.; Scheinfeld, N.; Ross, B. Topical antibacterial agents for wound care: A primer. Dermatol. Surg. 2003, 29, 620–626. [Google Scholar] [PubMed]
- Gehrig, K.A.; Warshaw, E.M. Allergic contact dermatitis to topical antibiotics: Epidemiology, responsible allergens, and management. J. Am. Acad. Dermatol. 2008, 58, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, L.; Chabal, C.; Brody, M.C. A dose-response study of intrathecal morphine: Efficacy, duration, optimal dose, and side effects. Anesth. Analg. 1988, 67, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Abd-Allah, A.R.; Aly, H.A.; Moustafa, A.M.; Abdel-Aziz, A.A.; Hamada, F.M. Adverse testicular effects of some quinolone members in rats. Pharmacol. Res. 2000, 41, 211–219. [Google Scholar] [CrossRef]
- Cottarel, G.; Wierzbowski, J. Combination drugs, an emerging option for antibacterial therapy. Trends Biotechnol. 2007, 25, 547–555. [Google Scholar] [CrossRef]
- Jackman, J.A.; Yoon, B.K.; Li, D.; Cho, N.J. Nanotechnology Formulations for Antibacterial Free Fatty Acids and Monoglycerides. Molecules 2016, 21, 305. [Google Scholar] [CrossRef] [Green Version]
- Gong, J.; Chen, M.; Zheng, Y.; Wang, S.; Wang, Y. Polymeric micelles drug delivery system in oncology. J. Control. Release 2012, 159, 312–323. [Google Scholar] [CrossRef]
- Zylberberg, C.; Matosevic, S. Pharmaceutical liposomal drug delivery: A review of new delivery systems and a look at the regulatory landscape. Drug Deliv. 2016, 23, 3319–3329. [Google Scholar] [CrossRef] [Green Version]
- Nanjwade, B.K.; Bechra, H.M.; Derkar, G.K.; Manvi, F.V.; Nanjwade, V.K. Dendrimers: Emerging polymers for drug-delivery systems. Eur. J. Pharm. Sci. 2009, 38, 185–196. [Google Scholar] [CrossRef]
- Chacko, R.T.; Ventura, J.; Zhuang, J.; Thayumanavan, S. Polymer nanogels: A versatile nanoscopic drug delivery platform. Adv. Drug Deliver. Rev. 2012, 64, 836–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, M.; Mordiffi, S.Z.; Lang, D. Effectiveness of polyhexamethylene biguanide impregnated dressing in wound healing: A systematic review protocol. JBI Database System Rev. Implement. Rep. 2016, 14, 76–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chindera, K.; Mahato, M.; Sharma, A.K.; Horsley, H.; Kloc-Muniak, K.; Kamaruzzaman, N.F.; Kumar, S.; McFarlane, A.; Stach, J.; Bentin, T.; et al. The antimicrobial polymer PHMB enters cells and selectively condenses bacterial chromosomes. Sci. Rep. 2016, 6, 23121. [Google Scholar] [CrossRef] [PubMed]
- Hubner, N.O.; Kramer, A. Review on the efficacy, safety and clinical applications of polihexanide, a modern wound antiseptic. Skin Pharmacol. Physiol. 2010, 23, 17–27. [Google Scholar] [CrossRef]
- Oule, M.K.; Azinwi, R.; Bernier, A.M.; Kablan, T.; Maupertuis, A.M.; Mauler, S.; Nevry, R.K.; Dembele, K.; Forbes, L.; Diop, L. Polyhexamethylene guanidine hydrochloride-based disinfectant: A novel tool to fight meticillin-resistant Staphylococcus aureus and nosocomial infections. J. Med. Microbiol. 2008, 57, 1523–1528. [Google Scholar] [CrossRef]
- Sibbald, R.G.; Coutts, P.; Woo, K.Y. Reduction of bacterial burden and pain in chronic wounds using a new polyhexamethylene biguanide antimicrobial foam dressing-clinical trial results. Adv. Skin Wound Care 2011, 24, 78–84. [Google Scholar] [CrossRef]
- Ahani, E.; Montazer, M.; Toliyat, T.; Mahmoudi Rad, M.; Harifi, T. Preparation of nano cationic liposome as carrier membrane for polyhexamethylene biguanide chloride through various methods utilizing higher antibacterial activities with low cell toxicity. J. Microencapsul. 2017, 34, 121–131. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, S.; Akhtar, N.; Ghauri, M.A.; Rajoka, M.I.; Khalid, Z.M.; Hussain, I. Polyhexamethylene biguanide functionalized cationic silver nanoparticles for enhanced antimicrobial activity. Nanoscale Res. Lett. 2012, 7, 267. [Google Scholar] [CrossRef] [Green Version]
- Cai, Z.; Zhang, H.; Wei, Y.; Wu, M.; Fu, A. Shear-thinning hyaluronan-based fluid hydrogels to modulate viscoelastic properties of osteoarthritis synovial fluids. Biomater. Sci. 2019, 7, 3143–3157. [Google Scholar] [CrossRef]
- Iqbal, M.; Tao, Y.; Xie, S.; Zhu, Y.; Chen, D.; Wang, X.; Huang, L.; Peng, D.; Sattar, A.; Shabbir, M.A.B.; et al. Aqueous two-phase system (ATPS): An overview and advances in its applications. Biol. Proced. Online 2016, 18, 18. [Google Scholar] [CrossRef] [Green Version]
- Qi, L.; Xu, Z.; Jiang, X.; Hu, C.; Zou, X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr. Res. 2004, 339, 2693–2700. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Pai, P.J.; Zhang, W.; Hu, Y.; Dong, X.; Qian, P.Y.; Chen, D.; Lam, H. Proteomic response of methicillin-resistant S. aureus to a synergistic antibacterial drug combination: A novel erythromycin derivative and oxacillin. Sci. Rep. 2016, 6, 19841. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Yu, H.; Zhang, C.; Chen, X.; Cheng, Z.; Bai, R.; Wu, X.; Yu, Q.; Wu, C.; Diao, Y. Nano-porous nitrocellulose liquid bandage modulates cell and cytokine response and accelerates cutaneous wound healing in a mouse model. Carbohyd. Polym. 2016, 136, 618–629. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |








| MIC(µg/mL) | PHMB | PVA/PHMB | CHT/PHMB | PVA/CHT/PHMB |
|---|---|---|---|---|
| S. aureus | 2 | 2 | 1 | 0.5 |
| E. coli | 2 | 1 | 1 | 0.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, Y.; Qian, Z.; Yin, Y.; Yuan, W.; Wu, F.; Jin, T. Polyvinyl Alcohol/Chitosan/Polyhexamethylene Biguanide Phase Separation System: A Potential Topical Antibacterial Formulation with Enhanced Antimicrobial Effect. Molecules 2020, 25, 1334. https://doi.org/10.3390/molecules25061334
Ni Y, Qian Z, Yin Y, Yuan W, Wu F, Jin T. Polyvinyl Alcohol/Chitosan/Polyhexamethylene Biguanide Phase Separation System: A Potential Topical Antibacterial Formulation with Enhanced Antimicrobial Effect. Molecules. 2020; 25(6):1334. https://doi.org/10.3390/molecules25061334
Chicago/Turabian StyleNi, Yunzhou, Zhixiang Qian, Yu Yin, Weien Yuan, Fei Wu, and Tuo Jin. 2020. "Polyvinyl Alcohol/Chitosan/Polyhexamethylene Biguanide Phase Separation System: A Potential Topical Antibacterial Formulation with Enhanced Antimicrobial Effect" Molecules 25, no. 6: 1334. https://doi.org/10.3390/molecules25061334