Tris(2-Aminoethyl)Amine/Metal Oxides Hybrid Materials—Preparation, Characterization and Catalytic Application
Abstract
:1. Introduction
2. Results and Discussion
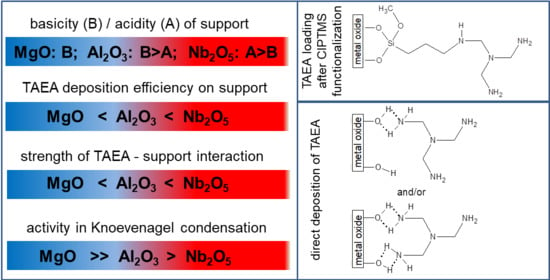
2.1. Characterization of Supports
2.2. Characterization of TAEA/Metal Oxide Composites
2.3. Knoevenagel Condensation between Furfural and Malononitrile
3. Materials and Methods
3.1. MgO, Al2O3 and Nb2O5 Modification Direct with Tris(2-aminoethyl)amine or after (3-chloropropyl)trimethoxysilane (ClPTMS) Anchoring
3.1.1. The Direct Modification of Metal Oxides with Tris(2-aminoethyl)amine
3.1.2. The Modification of Metal Oxides with tris(2-aminoethyl)amine after (3-chloropropyl)trimethoxysilane (ClPTMS) Anchoring
3.2. MgO Modification with (3-Aminopropyl)trimethoxysilane
3.3. Samples Characterization
3.4. Test Reactions
3.4.1. 2-Propanol Dehydration and Dehydrogenation
3.4.2. Dehydration and Cyclization of 2,5-Hexanedione
3.5. Knoevenagel Condensation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dalessandro, E.V.; Collin, H.P.; Guimarães, L.G.L.; Valle, M.S.; Pliego, J.R., Jr. Mechanism of the Piperidine-Catalyzed Knoevenagel Condensation Reaction in Methanol: The Role of Iminium and Enolate Ions. J. Phys. Chem. B 2017, 121, 5300–5307. [Google Scholar] [CrossRef]
- Khoshnavazi, R.; Bahrami, L.; Rezaei, M. Heteropolytungstostannate as a homo- and heterogeneous catalyst for Knoevenagel condensations, selective oxidation of sulfides and oxidative amination of aldehydes. RSC Adv. 2017, 7, 45495–45503. [Google Scholar] [CrossRef] [Green Version]
- Blasco-Jiménez, D.; Sobczak, I.; Ziolek, M.; López-Peinado, A.J.; Martín-Aranda, R.M. Amino-grafted metallosilicate MCM-41 materials as basic catalysts for eco-friendly processes. Catal. Today 2010, 152, 119–125. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, X.; Chen, S.-Y. Applications of Amine-functionalized Mesoporous Silica in Fine Chemical Synthesis. Top. Catal. 2009, 52, 681–687. [Google Scholar] [CrossRef]
- Olejniczak, M.; Ziolek, M. Comparative study of Zr, Nb, Mo containing SBA-15 grafted with amino-organosilanes. Microporous Mesoporous Mater. 2014, 196, 243–253. [Google Scholar] [CrossRef]
- Rajabi, F.; Fayyaz, F.; Luque, R. Cytosine-functionalized SBA-15 mesoporous nanomaterials: Synthesis, characterization and catalytic applications. Microporous Mesoporous Mater. 2017, 253, 64–70. [Google Scholar] [CrossRef]
- Sujandi; Park, S.-E.; Han, D.-S.; Han, S.-C.; Jin, M.-J.; Ohsuna, T. Amino-functionalized SBA-15 type mesoporous silica having nanostructured hexagonal platelet morphology. Chem. Commun. 2006, 4131–4133. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, K.S.K.; Chan, J.C.; Cheng, S. Preparation of ordered large pore SBA-15 silica functionalized with aminopropyl groups through one-pot synthesis. Chem. Commun. 2004, 2, 2762–2763. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Yang, D.; Zhang, F.; Li, H. Amine-bridged periodic mesoporous organosilica nanospheres as an active and reusable solid base-catalyst for water-medium and solvent-free organic reactions. J. Mol. Catal. A Chem. 2012, 387–397. [Google Scholar] [CrossRef]
- Zahouily, M.; Salah, M.; Bahlaouane, B.; Rayadh, A.; Houmam, A.; Hamed, E.A.; Sebti, S. Solid catalysts for the production of fine chemicals: The use of natural phosphate alone and doped base catalysts for the synthesis of unsaturated arylsulfones. Tetrahedron 2004, 60, 1631–1635. [Google Scholar] [CrossRef]
- Banerjee, R.; Mondal, S.; Purkayastha, P. Revival, enhancement and tuning of fluorescence from Coumarin 6: Combination of host–guest chemistry, viscosity and collisional quenching. RSC Adv. 2016, 6, 105347–105349. [Google Scholar] [CrossRef]
- Lai, S.M.; Martin-Aranda, R.; Yeung, K.L. Knoevenagel condensation reaction in zeolite membrane microreactor. Chem. Commun. 2003, 218–219. [Google Scholar] [CrossRef] [PubMed]
- Spallarossa, A.; Caneva, C.; Caviglia, M.; Alfei, S.; Butini, S.; Campiani, G.; Gemma, S.; Brindisi, M.; Zisterer, D.M.; Bright, S.A.; et al. Unconventional Knoevenagel-type indoles: Synthesis and cell-based studies for the identification of pro-apoptotic agents. Eur. J. Med. Chem. 2015, 102, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, H.S.; Sapra, S.; Gupta, M.; Nepali, K.; Gautam, R.; Yadav, S.; Kumar, R.; Jachak, S.M.; Chugh, M.; Gupta, M.K.; et al. Synthesis and biological evaluation of arylidene analogues of Meldrum’s acid as a new class of antimalarial and antioxidant agents. Bioorganic Med. Chem. 2010, 18, 5626–5633. [Google Scholar] [CrossRef]
- Adam, F.; Hello, K.M.; Osman, H. The heterogenation of melamine and its catalytic activity. Appl. Catal. A Gen. 2010, 382, 115–121. [Google Scholar] [CrossRef]
- Kryszak, D.; Stawicka, K.; Trejda, M. Calcium and nitrogen species loaded into SBA-15-a promising catalyst tested in Knoevenagel condensation. Dalt. Trans. 2020, 49, 9781–9794. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, H.; Tong, M.; Xiao, Y.; Li, H.; Zhang, F. Primary amine-functionalized mesoporous phenolic resin as an effective and stable solid base catalyst for Knoevenagel reactions in water. Green Synth. Catal. 2020, 1, 79–82. [Google Scholar] [CrossRef]
- Palraj, K.; Seo, Y.K.; Shim, K.E.; Hwang, Y.K.; Lee, U.H.; Hwang, D.W.; Hong, D.Y.; Halligudi, S.B.; Chang, J.S. Effect of diamine in amine-functionalized MIL-101 for Knoevenagel condensation. Bull. Korean Chem. Soc. 2011, 32, 2073–2075. [Google Scholar] [CrossRef] [Green Version]
- Wojtaszek-Gurdak, A.; Calvino-Casilda, V.; Grzesinska, A.; Martin-Aranda, R.; Ziolek, M. Impact of Brønsted acid sites in MWW zeolites modified with cesium and amine species on Knoevenagel condensation. Microporous Mesoporous Mater. 2019, 280, 288–296. [Google Scholar] [CrossRef]
- Li, G.; Xiao, J.; Zhang, W. Knoevenagel condensation catalyzed by a tertiary-amine functionalized polyacrylonitrile fiber. Green Chem. 2011, 13, 1828–1836. [Google Scholar] [CrossRef]
- Varadwaj, G.B.B.; Rana, S.; Parida, K.M. Amine functionalized K10 montmorillonite: A solid acid–base catalyst for the Knoevenagel condensation reaction. Dalton Trans. 2013, 42, 5122–5129. [Google Scholar] [CrossRef] [PubMed]
- Erigoni, A.; Hernández-Soto, M.C.; Rey, F.; Segarra, C.; Diaz, U. Highly active hybrid mesoporous silica-supported base organocatalysts for C C bond formation. Catal. Today 2020, 345, 227–236. [Google Scholar] [CrossRef]
- Gervasini, A.; Fenyvesi, J.; Auroux, A. Study of the acidic character of modified metal oxide surfaces using the test of isopropanol decomposition. Catal. Lett. 1997, 43, 219–228. [Google Scholar] [CrossRef]
- Dessau, R.M. Base- and acid-catalysed cyclization of diketones over ZSM-5. Zeolites 1990, 10, 205–206. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Lian, J.; Ma, J.; Duan, X.; Kim, T.; Li, H.; Zheng, W. One-step ionothermal synthesis of γ-Al2O3 mesoporous nanoflakes at low temperature. Chem. Commun. 2010, 46, 2650–2652. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-X.; Xu, D.; Li, X.-Q.; Liu, W.-C.; Jia, Y. Excellent fluoride removal properties of porous hollow MgO microspheres. New J. Chem. 2014, 38, 5445–5452. [Google Scholar] [CrossRef]
- Brandão, R.F.; Quirino, R.L.; Mello, V.M.; Tavares, A.P.; Peres, A.C.; Guinhos, F.; Rubim, J.C.; Suarez, P.A.Z. Synthesis, characterization and use of Nb2O5 based catalysts in producing biofuels by transesterification, esterification and pyrolysis. J. Braz. Chem. Soc. 2009, 20, 954–966. [Google Scholar] [CrossRef] [Green Version]
- Yousefi, S.; Ghasemi, B.; Tajally, M.; Asghari, A. Optical properties of MgO and Mg(OH)2 nanostructures synthesized by a chemical precipitation method using impure brine. J. Alloy. Compd. 2017, 711, 521–529. [Google Scholar] [CrossRef]
- Sutapa, I.W.; Wahab, A.W.; Taba, P.; La Nafie, N. Synthesis and Structural Profile Analysis of the MgO Nanoparticles Produced Through the Sol-Gel Method Followed by Annealing Process. Orient. J. Chem. 2018, 34, 1016–1025. [Google Scholar] [CrossRef] [Green Version]
- Stuart, B. Infrared Spectroscopy: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2004; ISBN 0470854278. [Google Scholar]
- Khazaei, A.; Nazari, S.; Karimi, G.; Ghaderi, E.; Mansouri Moradian, K.; Bagherpor, Z.; Nazari, S. Synthesis and characterization of γ-alumina porous nanoparticles from sodium aluminate liquor with two different surfactants. Int. J. Nanosci. Nanotechnol. 2016, 12, 207–214. [Google Scholar]
- Jin, X.; Liu, C.; Xu, J.; Wang, Q.; Chen, D. Size-controlled synthesis of mesoporous Nb2O5 microspheres for dye sensitized solar cells. RSC Adv. 2014, 4, 35546–35553. [Google Scholar] [CrossRef]
- Kryszak, D.; Stawicka, K.; Calvino-Casilda, V.; Martin-Aranda, R.; Ziolek, M. Imidazole immobilization in nanopores of silicas and niobiosilicates SBA-15 and MCF—A new concept towards creation of basicity. Appl. Catal. A Gen. 2017, 531, 139–150. [Google Scholar] [CrossRef]
- Kimmins, S.D.; Wyman, P.; Cameron, N.R. Amine-functionalization of glycidyl methacrylate-containing emulsion-templated porous polymers and immobilization of proteinase K for biocatalysis. Polymer (Guildford) 2014, 55, 416–425. [Google Scholar] [CrossRef] [Green Version]
- Appaturi, J.N.; Ramalingam, R.J.; Al-Lohedan, H.A. Synthesis, characterization and catalytic activity of melamine immobilized MCM-41 for condensation reactions. J. Porous Mater. 2018, 25, 629–641. [Google Scholar] [CrossRef]
- Martínez, F.; Orcajo, G.; Briones, D.; Leo, P.; Calleja, G. Catalytic advantages of NH2-modified MIL-53(Al) materials for Knoevenagel condensation reaction. Microporous Mesoporous Mater. 2017, 246, 43–50. [Google Scholar] [CrossRef]
- Appaturi, J.N.; Johan, M.R.; Ramalingam, R.J.; Al-Lohedan, H.A. Highly efficient green mesostructured urea functionalized on SBA-15 catalysts for selective synthesis of benzlidenemalononitrile. Microporous Mesoporous Mater. 2018, 256, 67–74. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |











| Catalyst | Conversion of 2,5-HDN (%) | Selectivity (%) | MCP/DMF | |
|---|---|---|---|---|
| MCP | DMF | |||
| MgO | 89 ± 0.4 | 0.4 ± 0.01 | 99.6 ± 0.01 | 249 |
| Al2O3 | 36 ± 0.2 | 7.0 ± 0.1 | 93.0 ± 0.1 | 13 |
| Nb2O5 | 8 ± 0.1 | 65.9 ± 0.9 | 34.1 ± 0.9 | 0.5 |
| Catalyst * | Amine Modifer, mmol/g (Nominal) |
|---|---|
| 3NH2 + MgO | 0.25 |
| 3NH2 + Al2O3 | 0.35 |
| 3NH2 + Nb2O5 | 0.50 |
| NH2/MgO | 0.47 |
| 3NH2/MgO | 0.62 |
| 3NH2/Al2O3 | 1.40 |
| 3NH2/Nb2O5 | 1.40 |
| Catalyst | Surface Area, m2/g | Pore Volume, cm3/g | Average Pore Diameter, nm (BJH) | Average Pore Diameter, nm (PSD) |
|---|---|---|---|---|
| MgO | 130.0 ± 0.9 | 0.29 | 7.86 | 5.9 |
| Al2O3 | 172.0 ± 1.3 | 0.47 | 8.49 | 10.2 |
| Nb2O5 | 123.0 ± 1.1 | 0.12 | 5.70 | 4.6/macropores |
| 3NH2 + MgO | 50 ± 0.5 | 0.17 | 13.26 | 5.9 |
| 3NH2 + Al2O3 | 146 ± 1.9 | 0.40 | 7.79 | 9.6 |
| 3NH2 + Nb2O5 | 40 ± 0.4 | 0.09 | 8.52 | 4.6/macropores |
| 3NH2/MgO | 69.9 ± 1.2 | 0.20 | 8.92 | 5.9 |
| 3NH2/Al2O3 | 29.3 ± 0.4 | 0.08 | 9.16 | 9.6 |
| Cl/Nb2O5 | 71.0 ± 0.8 | 0.08 | 7.40 | 4.6/macropores |
| 3NH2/Nb2O5 | 0.4 ± 0.01 | 0.0004 | 10.4 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stawicka, K.; Ziolek, M. Tris(2-Aminoethyl)Amine/Metal Oxides Hybrid Materials—Preparation, Characterization and Catalytic Application. Molecules 2020, 25, 4689. https://doi.org/10.3390/molecules25204689
Stawicka K, Ziolek M. Tris(2-Aminoethyl)Amine/Metal Oxides Hybrid Materials—Preparation, Characterization and Catalytic Application. Molecules. 2020; 25(20):4689. https://doi.org/10.3390/molecules25204689
Chicago/Turabian StyleStawicka, Katarzyna, and Maria Ziolek. 2020. "Tris(2-Aminoethyl)Amine/Metal Oxides Hybrid Materials—Preparation, Characterization and Catalytic Application" Molecules 25, no. 20: 4689. https://doi.org/10.3390/molecules25204689