Attraction of Adults of Cyclocephala lunulata and Cyclocephala barrerai (Coleoptera: Scarabaeoidea: Melolonthidae) towards Bacteria Volatiles Isolated from Their Genital Chambers
Abstract
:1. Introduction
2. Results and Discussion
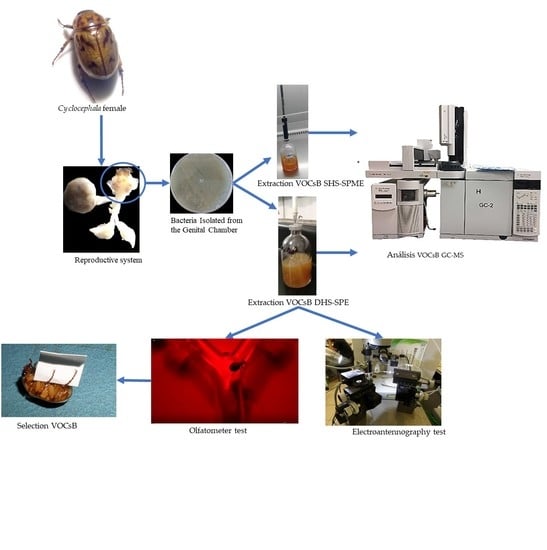
2.1. Bacteria Isolated from the Genital Chamber of Cyclocephala
2.2. Identification of VOCsB
2.3. Insect Attraction to VOCsB
2.4. Test of EAG
3. Materials and Methods
3.1. Insects
3.2. Microorganisms of the Female Reproductive System
3.3. Extraction and Identification of VOCsB
- VOCsB were captured by SHS-SPME, inserting a 65-μM PDMS/DVB fiber (Supelco, Inc., Bellefonte, PA, USA) into the bottle and maintaining the system for 16 h at 30 °C. After removing the fiber, the sample was analyzed by GC-MS. Ten repetitions were carried out for each bacterial strain in TSI; the control was a sterile TSI medium without bacteria.
- The capture of VOCsB by DHS-SPE was performed under the aforementioned conditions for bacterial growth in the TSI medium; cultures were incubated for a period of 8 h at 30 °C. Pasteur pipette with 125 mg of Super Q 80/100 (Alltech Assoc, Inc., Deerfield, IL, USA) was connected to the upper part of the flask through the silicone septa. Once the Pasteur pipette was inserted, the experiment was maintained for 16 h at 30 °C.
3.4. Bioassays
3.4.1. Olfactometer
3.4.2. Electroantennography
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jackson, T.A.; Klein, M.G. Scarabs as pests: A continuing problem. Coleopt. Bull. 2006, 60, 102–119. [Google Scholar] [CrossRef]
- Morón, M.Á.; Nogueira, G.; Rojas-Gómez, C.V.; Arce-Pérez, R. Biodiversidad de Melolonthidae (Coleoptera) en México. Rev. Mex. Biodivers. 2014, 85, 298–302. [Google Scholar] [CrossRef] [Green Version]
- Cherman, M.A.; Grossi, P.C. A crop pest species of Cyclocephala Dejean (Coleoptera: Melolonthidae: Dynastinae) misidentified for over twenty years in Southern Brazil. Bragantia 2020, 79, 372–376. [Google Scholar] [CrossRef]
- Ratcliffe, B. The Dynastinae scarab beetles of Costa Rica and Panama (Coleoptera: Scarabaeidae: Dynastinae). Bull. Univ. Neb. State Mus. 2003, 16, 506–527. [Google Scholar]
- Abarca, G.; Quesada, M. Especies del complejo de jobotos (Phyllophaga spp., Anomala spp. y Cyclocephala spp.) asociadas a cultivos en el Valle Central y Pacífico Seco de Costa Rica. Agron. Mesoam. 1997, 8, 44–53. [Google Scholar] [CrossRef]
- Stechauner-Rohringer, R. Redescripción de inmaduros, ciclo de vida, distribución e importancia agrícola de Cyclocephala lunulata Burmeister (Coleoptera: Melolonthidae: Dynastinae) en Colombia. Bol. Cient. Mus. Hist. Nat. 2010, 14, 203–220. [Google Scholar]
- Dicke, M.; Sabelis, M.W. Infochemical terminology: Based on cost benefit analysis rather origin of compounds. Funct. Ecol. 1988, 2, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Mitchell-Olds, T.; Gershenzon, J.; Baldwin, I.; Boland, W. Chemical ecology in the molecular era. Trends Plant Sci. 1998, 3, 362–365. [Google Scholar] [CrossRef]
- Robbins, P.S.; Crocker, R.L.; Nojima, S.; Morris, B.D.; Roelofs, W.L.; Villani, M.G. Methyl 2-(methylthio) benzoate: The unique sulfur-containing sex pheromone of Phyllophaga crinite. J. Insect Sci. 2003, 11, 517–520. [Google Scholar] [CrossRef] [Green Version]
- Romero-López, A.A.; Arzuffi, R. Evidencias sobre la producción y liberación de compuestos bioactivos de la feromona sexual de un melolóntido mexicano. In Ecología y Control de Plagas Edafícolas; Rodríguez del Bosque, L.A., Morón, M.A., Eds.; Publicación especial del Instituto de Ecología AC: Xalapa, Mexico, 2010; Volume 1, pp. 204–222. [Google Scholar]
- Leal, W.S. Chemical ecology of phytagous scarab beetles. Annu. Rev. Entomol. 1998, 43, 39–61. [Google Scholar] [CrossRef] [Green Version]
- Facundo, H.T.; Linn Jr., C.E.; Villani, M.G.; Roelofs, W.L. Emergence, mating, and postmating behaviors of the Oriental beetle (Coleoptera: Scarabaeidae). J. Insect Behav. 1999, 12, 175–192. [Google Scholar] [CrossRef]
- Benítez-Herrera, L.N.; Martínez, I.; Romero-López, A.A. Anatomía del aparato reproductor de Macrodactylus mexicanus (Coleoptera: Scarabaeoidea: Melolonthidae) y su posible participación en su comunicación química sexual. Southwest. Entomol. 2015, 40, 189–198. [Google Scholar] [CrossRef]
- Romero-López, A.A.; Arzuffi, R.; Valdez, J.; Sánchez-Espíndola, E.; Morón, M.A. Tissues involved in sex pheromone production in Phyllophaga obsoleta (Coleoptera: Scarabaeoidea: Melolonthidae). Ann. Entomol. Soc. Am. 2011, 104, 960–965. [Google Scholar] [CrossRef] [Green Version]
- Hoyt, C.P.; Osborne, G.O.; Mulcock, A.P. Production of an insect sex attractant by symbiotic bacteria. Nature 1971, 230, 472–473. [Google Scholar] [CrossRef] [PubMed]
- Marshall, D.G.; Jackson, T.A.; Unelius, C.R.; Wee, S.L.; Young, S.D.; Townsend, R.J.; Suckling, D.M. Morganella morganii bacteria produces phenol as the sex pheromone of the New Zealand grass grub from tyrosine in the colleterial gland. Sci. Nat. 2016, 103, 59. [Google Scholar] [CrossRef]
- Rosete-Enríquez, M.; Romero-López, A.A. Klebsiella bacteria isolated from the genital chamber of Phyllophaga obsoleta 1. Southwest. Entomol. 2017, 42, 1003–1013. [Google Scholar] [CrossRef]
- Schulz, S.; Dickschat, J.S. Bacterial volatiles: The smell of small organisms. Nat. Prod. Rep. 2007, 24, 814–842. [Google Scholar] [CrossRef]
- Kai, M.; Haustein, M.; Molina, F.; Petri, A.; Scholz, B.; Piechulla, B. Bacterial volatiles and their action potential. App. Microbiol. Biotechnol. 2009, 81, 1001–1012. [Google Scholar] [CrossRef]
- Turton, L.J.; Drucker, D.B.; Ganguli, L.A. Effect of glucose concentration in the growth medium upon neutral and acidic fermentation end-products of Clostridium bifermentans, Clostridium sporogenes and Peptostreptococcus anaerobius. J. Med. Microbiol. 1983, 16, 61–67. [Google Scholar] [CrossRef]
- Gao, C.; Wang, A.; Wu, W.M.; Yin, Y.; Zhao, Y.G. Enrichment of anodic biofilm inoculated with anaerobic or aerobic sludge in single chambered air-cathode microbial fuel cells. Bioresour. Technol. 2014, 167, 124–132. [Google Scholar] [CrossRef]
- Jia, B.; Sohnlein, B.; Mortelmans, K.; Coggiola, M.; Oser, H. Distinguishing Methicillin resistant and sensitive Staphylococcus aureus using volatile headspace metabolites. IEEE. Sens. J. 2010, 10, 71–75. [Google Scholar] [CrossRef]
- Rowan, D.D. Volatile metabolites. Metabolites 2011, 1, 41–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tait, E.; Perry, J.D.; Stanforth, S.P.; Dean, J.R. Identification of volatile organic compounds produced by bacteria using HS-SPME-GC-MS. J. Chromatogr. Sci. 2014, 52, 363–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elgaali, H.; Hamilton-Kemp, T.R.; Newman, M.C.; Collins, R.W.; Yu, K.; Archbold, D.D. Comparison of long-chain alcohols and other volatile compounds emitted from food-borne and related Gram positive and Gram negative bacteria. J. Basic. Microbiol. 2002, 42, 373–380. [Google Scholar] [CrossRef]
- Hamilton-Kemp, T.; Newman, M.; Collins, R.; Elgaali, H.; Yu, K.; Archbold, D. Production of the long-chain alcohols octanol, decanol, and dodecanol by Escherichia coli. Curr. Microbiol. 2005, 51, 82–86. [Google Scholar] [CrossRef]
- Thibout, E.; Guillot, J.F.; Auger, J. Microorganisms are involved in the production of volatile kairomones affecting the host seeking behaviour of Diadromus pulchellus, a parasitoid of Acrolepiopsis assectella. Physiol. Entomol. 1993, 18, 176–182. [Google Scholar] [CrossRef]
- Verhulst, N.O.; Beijleveld, H.; Knols, B.G.; Takken, W.; Schraa, G.; Bouwmeester, H.J.; Smallegange, R.C. Cultured skin microbiota attracts malaria mosquitoes. Malar. J. 2009, 8, 302. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.J.; Chaudhury, M.F.; Durso, L.M.; Sagel, A.; Skoda, S.R.; Jelvez-Serra, N.S.; Santanab, E.G. Semiochemicals released from five bacteria identified from animal wounds infested by primary screwworms and their effects on fly behavioral activity. PLoS ONE 2017, 12, e0179090. [Google Scholar] [CrossRef]
- Blomquist, G.J.; Figueroa-Teran, R.; Aw, M.; Song, M.; Gorzalski, A.; Abbott, N.L.; Tittiger, C. Pheromone production in bark beetles. Insect Biochem. Mol. 2010, 40, 699–712. [Google Scholar] [CrossRef]
- Thacker, J.R.; Train, M.R. Use of volatiles in pest control. Chem. Biol. Volatiles 2010, 5, 151–172. [Google Scholar] [CrossRef]
- Davis, T.S.; Crippen, T.L.; Hofstetter, R.W.; Tomberlin, J.K. Microbial Volatile Emissions as Insect Semiochemicals. J. Chem. Ecol. 2013, 30, 840–859. [Google Scholar] [CrossRef] [PubMed]
- Robacker, D.C.; Lauzon, C.R.; He, X. Volatiles production and attractiveness to the Mexican fruit fly of Enterobacter agglomerans isolated from apple maggot and Mexican fruit flies. J. Chem. Ecol. 2004, 30, 1329–1347. [Google Scholar] [CrossRef] [PubMed]
- Naaz, N.; Choudhary, J.S.; Prabhakar, C.S.; Moanaro; Maurya, S. Identification and evaluation of cultivable gut bacteria associated with peach fruit fly, Bactrocera zonata (Biptera: Tephritidae). Phytoparasitica 2016, 44, 165–176. [Google Scholar] [CrossRef]
- Dillon, R.; Vennard, C.; Charnley, A. A Note: Gut bacteria produce components of a locust cohesion pheromone. J. Appl. Microbiol. 2002, 92, 759–763. [Google Scholar] [CrossRef]
- Wada-Katsumata, A.; Zurek, L.; Nalyanya, G.; Roelofs, W.L.; Zhang, A.; Schal, C. Gut bacteria mediate aggregation in the German cockroach. Proc. Natl. Acad. Sci. USA 2015, 112, 15678–15683. [Google Scholar] [CrossRef] [Green Version]
- Hallett, R.H.; Perez, A.L.; Gries, G.; Gries, R.; Pierce Jr, H.D.; Yue, J.; Oehlschlager, A.C.; Gonzalez, L.M.; Borden, J.H. Aggregation pheromone of coconut rhinoceros beetle, Oryctes rhinoceros (L.) (Coleoptera: Scarabaeidae). J. Chem. Ecol. 1995, 21, 1549–1570. [Google Scholar] [CrossRef]
- Tolasch, T.; Solter, S.; Tóth, M.; Ruther, J.; Francke, W. (R)-acetoin-female sex pheromone of the summer chafer Amphimallon solstitiale (L.). J. Chem. Ecol. 2003, 29, 1045–1050. [Google Scholar] [CrossRef]
- Rochat, D.; Ramirez Lucas, P.; Malosse, C.; Aldana, R.; Kakul, T.; Morin, J.P. Role of solid-phase microextraction in the identification of highly volatile pheromones of two Rhinoceros beetles Scapanes australis and Strategus aloeus (Coleoptera, Scarabaeidae, Dynastinae). J. Chromatogr. 2000, 885, 433–444. [Google Scholar] [CrossRef]
- Oike, M.; Kanayama, S.; Wakamura, S. Mate-searching behavior of the black chafer Holotrichia kiotonensis (Coleoptera: Scarabaeidae): Identification of a sex pheromone, and male orientation behavior controlled by olfactory and visual cues. Appl. Entomol. Zool. 2017, 52, 519–526. [Google Scholar] [CrossRef]
- Ruther, J.; Reinecke, A.; Hilker, M.; Tolasch, T. Phenol—another cockchafer attractant shared by Melolontha hippocastani fabr. and M. melolontha L. Z. Fur Naturforsch-Sect. C J. Biosci. 2002, 57, 910–913. [Google Scholar] [CrossRef]
- Robacker, D.C.; Bartelt, R.J. Chemical attractive Mexican fruit fly from Klebsiella pneumoniae and Citrobacter freundii cultures sampled by solid-phase microextraction. J. Chem. Ecol. 1997, 23, 2897–2915. [Google Scholar] [CrossRef]
- Alves, M.S.; da Silva Dias, R.C.; de Castro, A.C.D.; Riley, L.W.; Moreira, B.M. Identification of clinical isolates of indole-positive and indole-negative Klebsiella spp. J. Clin. Microbiol. 2006, 44, 3640–3646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lü, Z.X.; Song, W. Research of indole-3-acetic acid biosynthetic pathway of Klebsiella oxytoca SG-11 by HPLC and GC-MS. Sepu Chin. J. Chromatogr. 2000, 18, 328. [Google Scholar]
- Xu, T.; Wang, C.; Zhu, S.; Zheng, G. Enzymatic preparation of optically pure t-butyl 6-chloro-(3R, 5S)-dihydroxyhexanoate by a novel alcohol dehydrogenase discovered from Klebsiella oxytoca. Process Biochem. 2017, 57, 72–79. [Google Scholar] [CrossRef]
- Engel, E.; Baty, C.; LeCorre, D.; Souchon, I.; Martin, N. Flavor-active compounds potentially implicated in cooked cauliflower acceptance. J. Agric. Food Chem. 2002, 50, 6459–6467. [Google Scholar] [CrossRef]
- Hayes, R.A.; Richardson, B.J.; Claus, S.C.; Wyllie, S.G. Semiochemicals and social signaling in the wild European rabbit in Australia: II. Variations in chemical composition of chin gland secretion across sampling sites. J. Chem. Ecol. 2002, 28, 2613–2625. [Google Scholar] [CrossRef]
- Ong, P.K.C.; Acree, T.E. Similarities in the aroma chemistry of Gewürztraminer variety wines and Lychee (Litchi chinesis Sonn.) fruit. J. Agric. Food Chem. 1999, 47, 665–670. [Google Scholar] [CrossRef]
- Farkas, O.; Heberger, K. Comparison of ridge regression, partial least-squares, pairwise correlation, forward and best subset selection methods for prediction of retention indices for aliphatic alcohols. J. Chem. Inf. Model. 2005, 45, 339–346. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 1995. [Google Scholar]
- Bendimerad, N.; Bendiab, S.A.T.; Benabadji, A.B.; Fernandez, X.; Valette, L.; Lizzani-Cuvelier, L. Composition and antibacterial activity of Pseudocytisus integrifolius (Salisb.) essential oil from Algeria. J. Agric. Food Chem. 2005, 53, 2947–2952. [Google Scholar] [CrossRef]
- Aznar, M.; Lopez, R.; Cacho, J.; Ferreira, V. Identification and quantification of impact odorants of aged red wines from Rioja. GC-olfactometry, quantitative GC-MS, and odor evaluation of HPLC fractions. J. Agric. Food Chem. 2001, 49, 2924–2929. [Google Scholar] [CrossRef]
- De Marques, F.A.; McElfresh, J.S.; Millar, J.G. Kováts retention indexes of monounsaturated C12, C14, and C16 alcohols, acetates and aldehydes commonly found in lepidopteran pheromone blends. J. Braz. Chem. Soc. 2000, 11, 592–599. [Google Scholar] [CrossRef]
- Tellez, M.R.; Schrader, K.K.; Kobaisy, M. Volatile components of the cyanobacterium Oscillatoria perornata (Skuja). J. Agric. Food Chem. 2001, 49, 5989–5992. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Cho, B.R.; Hahn, J.S. Metabolic engineering of Saccharomyces cerevisiae for the production of 2-phenylethanol via Ehrlich pathway. Biotechnol. Bioeng. 2014, 111, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Henzell, R.F.; Lowe, M.D. Sex attractant of the grass grub beetle. Science 1970, 168, 1005–1010. [Google Scholar] [CrossRef]
- Harrison, R.A. Grass grub attractant identified. N. Z. J. Agric. Res. 1970, 121, 25. [Google Scholar]
- Zarbin, P.H.G.; Leal, W.S.; Ávila, C.J.; Oliveira, L.J. Identification of the sex pheromone of Phyllophaga cuyabana (Coleoptera: Melolonthidae). Tetrahedron Lett. 2007, 48, 1991–1992. [Google Scholar] [CrossRef] [Green Version]
- Gruner, L.; Marival, D. The attraction of males of the West Indian beetle Cyclocephala insulicola Arrow by phenol (Coleoptera: Dynastinae). C.R. Acad. Seances Agric. Fr. 1974, 60, 203–208. [Google Scholar]
- Zhang, A.; Robbins, S.A.; Leal, W.S.; Lin, C., Jr.; Villani, M.G.; Roelofs, W.L. Essential amino acid methyl esters: Major sex pheromone components of the cranberry white grub, Phyllophaga anxia (Coleoptera: Scarabaeidae). J. Chem. Ecol. 1997, 23, 231–245. [Google Scholar] [CrossRef] [Green Version]
- Yasui, H.; Wakamura, S.; Tanaka, S.; Harano, K.; Mochizuki, F.; Nagayama, A.; Hokama, Y.; Arakaki, N. Quantification of 2-butanol as a sex attractant pheromone and related alcohols emitted by individual white grub beetle, Dasylepida ishigakiensis (Coleoptera: Scarabaeidae). App. Entomol. Zool. 2010, 45, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Ju, Q.; Guo, X.; Li, X.; Jiang, X.; Jiang, X.; Ni, W.; Qu, M. Plant volatiles increase sex pheromone attraction of Holotrichia parallela (Coleoptera: Scarabaeoidea). J. Chem. Ecol. 2017, 43, 236–242. [Google Scholar] [CrossRef]
- Cardé, R.T. Defining attraction and aggregation pheromones: Teleological versus functional perspectives. J. Chem. Ecol. 2014, 40, 519–520. [Google Scholar] [CrossRef] [PubMed]
- Toffin, E.; Gabriel, E.; Louis, M.; Deneubourg, J.; Grégoire, J. Colonization of weakened trees by mass-attacking bark beetles: No penalty for pioneers, scattered initial distributions and final regular patterns. R. Soc. Open Sci. 2018, 5, 170454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gries, G.; Gries, R.; Perez, A.L.; Oehlschlager, A.C.; Gonzales, L.M.; Pierce, H.D.; Zebeyou, M.; Kouame, B.; Kouame, B. Aggregation pheromone of the african rhinoceros beetle, Oryctes monoceros (Olivier) (Coleoptera: Scarabaeidae) b-binding site of photosystem ii. Z. Fur Naturforsch.-Sect. C J. Biosci. 1994, 49, 363–366. [Google Scholar] [CrossRef]
- Morin, J.P.; Rochat, D.; Malosse, C.; Lettere, M.; De Chenon, R.D.; Wibwo, H.; Descoins, C. Ethyl 4-methyloctanoate, major component of Oryctes rhinoceros (L.) (Coleoptera, Dynastidae) male pheromone. C.R. Acad. Sci. Paris. 1996, 319, 595–602. [Google Scholar] [PubMed]
- Rochat, D.; Mohammadpoor, K.; Malosse, C.; Avand-Faghih, A.; Lettere, M.; Beauhaire, J.; Morin, J.P.; Pezier, A.; Renou, M.; Abdollahi, G.A. Male aggregation pheromone of date palm fruit stalk borer Oryctes elegans. J. Chem. Ecol. 2004, 30, 387–407. [Google Scholar] [CrossRef] [PubMed]
- Allou, K.; Morin, J.P.; Kouassi, P.; N’klo, F.H.; Rochat, D. Oryctes monoceros trapping with synthetic pheromone and palm material in Ivory coast. J. Chem. Ecol. 2006, 32, 1743–1754. [Google Scholar] [CrossRef]
- Said, I.; Hasni, N.; Abdallah, Z.; Couzi, P.; Ouhichi, M.; Renou, M.; Rochat, D. Identification of the aggregation pheromone of the date palm root borer Oryctes agamemnon. J. Chem. Ecol. 2015, 41, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Leal, W.S.; Yadava, C.P.S.; Vijayvergia, J.N. Aggregation of the scarab beetle Holotrichia consanguinea in response to female-released pheromone suggests secondary function hypothesis for semiochemical. J. Chem. Ecol. 1996, 22, 1557–1566. [Google Scholar] [CrossRef]
- Arakaki, N.; Wakamura, S.; Yasui, H.; Sadoyama, Y.; Kishita, M. Sexually differentiated functions of female-produced pheromone of the black chafer Holotrichia loochooana loochooana (Sawada) (Coleoptera: Scarabaeidae). Chemoecology 2003, 13, 183–186. [Google Scholar] [CrossRef]
- Hunt, D.W.A.; Borden, J.H. Conversion of verbenols to verbenone by yeasts isolated from Dendroctonus ponderosae (Coleoptera: Scolytidae). J. Chem. Ecol. 1990, 16, 1385–1397. [Google Scholar] [CrossRef]
- Kubo, I.; Muroi, H.; Kubo, A. Structural functions of antimicrobial long-chain alcohols and phenols. Bioorg. Med. Chem. 1995, 3, 873–880. [Google Scholar] [CrossRef]
- Ruiu, L. Insect Pathogenic Bacteria in Integrated Pest Management. Insects 2015, 6, 352–367. [Google Scholar] [CrossRef] [Green Version]
- Morón, M.A.; Ratcliffe, B.C.; Deloya, C. Atlas de los Escarabajos de México. Coleoptera: Lamellicornia. Vol. I. Familia Melolonthidae; Publicación especial de la Sociedad Mexicana de Entomología, A.C. y Conabio: México, Mexico, 1997. [Google Scholar]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2017, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, E.G. Isolation and identification of drugs. Pharm. Press 1978, 2, 927–928. [Google Scholar]
Sample Availability: Samples are not available from the authors. |





| Melolonthidae Specie | Strain | Closest Matches | Accession no. | Similarity (%) | Phylogenetic Affiliation | Host/Isolation Source |
|---|---|---|---|---|---|---|
| Cyclocephala lunulata | Klebsiella sp. MT239565 | Klebsiella spallanzanii strain SPARK1058C2 | MN091365.1 | 100 | Enterobacteriaceae | Homo sapiens (urine) |
| Klebsiella grimontii strain SS141 | CP044527.1 | 100 | Enterobacteriaceae | Coffee cup | ||
| Klebsiella oxytoca strain 4928STDY7387762 | LR607363.1 | 100 | Enterobacteriaceae | Feces | ||
| Morganella morganii MT239566 | Morganella morganii strain FC6853 | MH628232.1 | 100 | Enterobacteriaceae | Homo sapiens (patient) | |
| Uncultured organism clone ELU0075-T355-S-NIPCRAMgANa_000386 | HQ774639.1 | 100 | Enterobacteriaceae | Homo sapiens (gastrointestinal) | ||
| Morganella morganii subsp. morganii strain JY 16S | KR094121.1 | 100 | Enterobacteriaceae | Medical College, Soochow University | ||
| Cyclocephala barrerai | Citrobacter freundii Cyl_Citf01 MG652605 | Citrobacter freundii strain FDAARGOS_61 | CP026045 | 99 | Enterobacteriaceae | Homo sapiens (rectal swab) |
| Citrobacter freundii strain BD | CP018810 | 99 | Enterobacteriaceae | Bactrocera dorsalis (gut) | ||
| Citrobacter sp. PT2B | GU458283 | 99 | Enterobacteriaceae | Reticulitermes speratus (gut) | ||
| Klebsiella oxytoca Cyl_Kleb02 MG652606 | Uncultured bacterium | JF208909 | 100 | Enterobacteriaceae | Homo sapiens (skin) | |
| Klebsiella oxytoca strain CAU9419 | MF428632 | 99 | Enterobacteriaceae | pickle | ||
| Klebsiella oxytoca strain NGB-FR100 | LC049195 | 99 | Enterobacteriaceae | Root nodules of fava bean |
| Morphology | Cyclocephala lunulata | Cyclocephala barrerai | ||
|---|---|---|---|---|
| Klebsiella sp. | Morganella morganii | Klebsiella oxytoca | Citrobacter freundii | |
| Shape | Circular | Circular | Circular | Circular |
| Edges | Rounded | Rounded | Rounded | Rounded |
| Elevation | Convex | Convex | Convex | Convex |
| Surface | Smooth | Smooth | Smooth | Smooth |
| Consistency | Creamy | Creamy | Creamy | Creamy |
| Pigmentation | Beige | Yellow | Beige | Yellow |
| Transmitted light | Translucent | Translucent | Translucent | Translucent |
| Optical property | Iridescent | Brilliant | Iridescent | Brilliant |
| Size (mm) | 1 | 1 | 1−2 | 1 |
| Individual morphology | bacillus | bacillus | bacillus | bacillus |
| Gram’s reaction | Negative | Negative | Negative | Negative |
| Specie Bacteria | Pk No. | Extraction Technique | VOCsB | KRI | Ref. KRI | Characteristic EI ions |
|---|---|---|---|---|---|---|
| Klebsiella sp. | 1 | SHS-SPME | (methyl disulfanyl) methane | 691 | 735 [46] | 45, 61, 69, 79,83, 91, 94 (M+) |
| 2 | SHS-SPME | (methyl trisulfanyl) methane | 966 | 961 [46] | 64, 79, 83, 111, 113, 126 (M+) | |
| 3 | SHS-SPME | phenol | 977 | 980 [47] | 40, 55, 66, 74, 94 (M+) | |
| 4 | SHS-SPME | 2-phenylethanol | 1087 | 1078 [48] | 51, 65, 77, 91, 92, 122 (M+) | |
| 5 | SHS-SPME | decan-1-ol | 1234 | 1256 [49] | 43, 70, 83, 97, 112, 140, 158 (M+) | |
| 6 | SHS-SPME | indole | 1251 | 1288 [50] | 40, 58, 63, 74, 90, 102, 117 (M+) | |
| 7 | SHS-SPME | tridecan-2-one | 1475 | 1474 [51] | 43, 58, 71, 85, 96, 140, 198 (M+) | |
| Morganella morganii | 1 | SHS-SPME | (methyl disulfanyl) methane | 691 | 735 [46] | 45, 61, 69, 79,83, 91, 94 (M+) |
| 2 | SHS-SPME/DHS-SPE | (methyl trisulfanyl) methane | 966 | 961 [46] | 64, 79, 83, 111, 113, 126 (M+) | |
| 3 | SHS-SPME/DHS-SPE | phenol | 977 | 980 [47] | 40, 55, 66, 74, 94 (M+) | |
| 4 | SHS-SPME/DHS-SPE | 2-phenylethanol | 1087 | 1078 [48] | 51, 65, 77, 91, 92, 122 (M+) | |
| 5 | SHS-SPME | decan-1-ol | 1234 | 1256 [49] | 43, 70, 83, 97, 112, 140, 158 (M+) | |
| 6 | SHS-SPME | indole | 1251 | 1288 [50] | 40, 58, 63, 74, 90, 102, 117 (M+) | |
| 7 | SHS-SPME | undecan-1-ol | 1282 | 1372 [51] | 55, 69, 83, 97, 111, 154, 172 (M+) | |
| Klebsiella oxytoca | 1 | SHS-SPME | 3-methylbutan-1-ol | 688 | 734 [50] | 42, 55, 57, 70, 87, 88 (M+) |
| 2 | SHS-SPME/DHS-SPE | 2-phenylethanol | 1087 | 1078 [48] | 51, 65, 77, 91, 92, 122 (M+) | |
| 3 | SHS-SPME | ethyl octanoate | 1179 | 1192 [52] | 57, 88, 127, 172 (M+) | |
| 4 | SHS-SPME | decan-1-ol | 1234 | 1256 [49] | 43, 70, 83, 97, 112, 140, 158 (M+) | |
| 5 | SHS-SPME | indole | 1251 | 1288 [50] | 40, 58, 63, 74, 90, 102, 117 (M+), | |
| 6 | SHS-SPME | dodecan-1-ol | 1461 | 1473 [53] | 69,83, 97,111, 140, 168, 186 (M+) | |
| 7 | SHS-SPME | tridecan-2-one | 1475 | 1474 [51] | 58, 71, 140, 169, 183, 198 (M+) | |
| Citrobacter freundii | 1 | SHS-SPME | (methyldisulfanyl) methane | 691 | 735 [46] | 45, 61, 69, 79,83, 91, 94 (M+) |
| 2 | SHS-SPME/DHS-SPE | (methyltrisulfanyl) methane | 966 | 961 [46] | 64, 79, 83, 111, 113, 126 (M+) | |
| 3 | SHS-SPME/DHS-SPE | phenol | 977 | 980 [47] | 40, 55, 66, 74, 94 (M+) | |
| 4 | SHS-SPME/DHS-SPE | 2-phenylethanol | 1087 | 1078 [48] | 51, 65, 77, 91, 92, 122 (M+) | |
| 5 | SHS-SPME | ethyl octanoate | 1179 | 1192 [52] | 57, 88, 127, 143, 157, 172 (M+) | |
| 6 | SHS-SPME/DHS-SPE | (methyltetrasulfanyl) methane | 1204 | 1210 [54] | 45, 64, 79, 94, 111, 145, 158 (M+) | |
| 7 | SHS-SPME | decan-1-ol | 1234 | 1256 [49] | 43, 70, 83, 97, 112, 140, 158 (M+) | |
| 8 | SHS-SPME | undecan-1-ol | 1282 | 1372 [51] | 55, 69, 83, 97, 111, 154, 172 (M+) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez-Cruz, A.; Robledo, N.; Rosete-Enríquez, M.; Romero-López, A.A. Attraction of Adults of Cyclocephala lunulata and Cyclocephala barrerai (Coleoptera: Scarabaeoidea: Melolonthidae) towards Bacteria Volatiles Isolated from Their Genital Chambers. Molecules 2020, 25, 4430. https://doi.org/10.3390/molecules25194430
Sanchez-Cruz A, Robledo N, Rosete-Enríquez M, Romero-López AA. Attraction of Adults of Cyclocephala lunulata and Cyclocephala barrerai (Coleoptera: Scarabaeoidea: Melolonthidae) towards Bacteria Volatiles Isolated from Their Genital Chambers. Molecules. 2020; 25(19):4430. https://doi.org/10.3390/molecules25194430
Chicago/Turabian StyleSanchez-Cruz, Abraham, Norma Robledo, María Rosete-Enríquez, and Angel A. Romero-López. 2020. "Attraction of Adults of Cyclocephala lunulata and Cyclocephala barrerai (Coleoptera: Scarabaeoidea: Melolonthidae) towards Bacteria Volatiles Isolated from Their Genital Chambers" Molecules 25, no. 19: 4430. https://doi.org/10.3390/molecules25194430