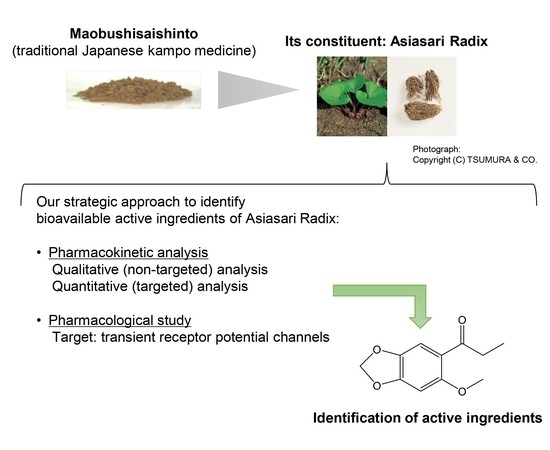
In Vivo Pharmacokinetic Analysis Utilizing Non-Targeted and Targeted Mass Spectrometry and In Vitro Assay against Transient Receptor Potential Channels of Maobushisaishinto and Its Constituent Asiasari Radix
Abstract
:1. Introduction
2. Results and Discussion
2.1. Qualitative (Non-Targeted) Analysis of AR Compounds in Plasma after AR or MBST Administration
2.2. Quantitative (Targeted) Analysis AR Ingredients in Plasma after MBST Administration
2.3. Bioavailable Ingredients Derived from AR Did Not Affect Excessive NO Production That Is Involved in Inflammation
2.4. Pharmacological Evaluation against TRP Channels of Bioavailable Ingredients Derived from MBST
3. Materials and Methods
3.1. Drugs and Reagents
3.2. Animals
3.3. Test Drug Doses in Rats
3.4. Plasma Sample Collection Following AR Administration
3.5. Plasma Sample Collection Following MBST Administration
3.6. Extract Sample Collection for the Quantification of the Contents of AR Ingredients in MBST
3.7. Qualitative (Non-Targeted) Analysis of AR Components in Plasma after AR or MBST Administration
3.8. Quantitative (Targeted) Analysis of AR Components in Rat Plasma after MBST Administration
3.9. Pharmacological Study
3.9.1. Construction of TRP Channel-Expressing Cells
3.9.2. Ca2+ Influx Assay for Evaluation of TRP Channel Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jamieson, D.J.; Theiler, R.; Rasmussen, S.A. Emerging Infections and Pregnancy. Emerg. Infect. Dis. 2006, 12, 1638–1643. [Google Scholar] [CrossRef]
- Weiskopf, D.; Weinberger, B.; Grubeck-Loebenstein, B. The aging of the immune system. Transpl. Int. 2009, 22, 1041–1050. [Google Scholar] [CrossRef]
- Ferber, D. ANTIBIOTIC RESISTANCE: Superbugs on the Hoof? Science 2000, 288, 792–794. [Google Scholar] [CrossRef] [Green Version]
- Aarestrup, F.M. Veterinary Drug Usage and Antimicrobial Resistance in Bacteria of Animal Origin. Basic Clin. Pharmacol. Toxicol. 2005, 96, 271–281. [Google Scholar] [CrossRef]
- Cabello, F.C. Heavy use of prophylactic antibiotics in aquaculture: A growing problem for human and animal health and for the environment. Environ. Microbiol. 2006, 8, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Cunha, B.A. Antibiotic Side Effects. Med. Clin. N. Am. 2001, 85, 149–185. [Google Scholar] [CrossRef]
- Heta, S.; Robo, I. The Side Effects of the Most Commonly Used Group of Antibiotics in Periodontal Treatments. Med. Sci. 2018, 6, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willyard, C. The drug-resistant bacteria that pose the greatest health threats. Nature 2017, 543, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Webster, T.J. Bacteria antibiotic resistance: New challenges and opportunities for implant-associated orthopedic infections. J. Orthop. Res. 2017, 36, 22–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higaki, S.; Morimatsu, S.; Morohashi, M.; Yamagishi, T.; Hasegawa, Y. Susceptibility of Propionibacterium acnes, Staphylococcus aureus and Staphylococcus epidermidis to 10 Kampo Formulations. J. Int. Med. Res. 1997, 25, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Minami, M.; Takase, H.; Nakamura, M.; Makino, T. Methanol extract of Lonicera caerulea var. emphyllocalyx fruit has antibacterial and anti-biofilm activity against Streptococcus pyogenes in vitro. Biosci. Trends 2019, 13, 145–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, T.; Kaneko, A.; Koseki, J.; Matsubara, Y.; Aiba, S.; Yamasaki, K. Pharmacokinetic Study of Bioactive Flavonoids in the Traditional Japanese Medicine Keigairengyoto Exerting Antibacterial Effects against Staphylococcus aureus. Int. J. Mol. Sci. 2018, 19, 328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwasaki, K.; Taguchi, M.; Cyong, J.C.; Akiba, T. Effects of mao-bushi-saishin-to on influenza vaccination in elderly subjects; a randomized control study. Kampo Immuno-Allergy 2004, 17, 97–103. [Google Scholar]
- Terashima, Y.; Hamazaki, K.; Itomura, M.; Huan, M.; Shibahara, N.; Kobayashi, S.; Hamazaki, T. Effect of a traditional Chinese medicine, maobushisaishinto, on the antibody titer after influenza vaccination: A randomized, placebo-controlled, double-blind trial. J. Trad. Med. 2007, 24, 59–66. [Google Scholar] [CrossRef]
- Takagi, Y.; Maeda, A.; Ueba, N.; Higashi, N.; Yamamura, H.; Nunoura, Y. Effect of Mao-bushi-saishin-to on the primary antibody response in mice of different ages. J. Trad. Med. 1996, 13, 94–99. [Google Scholar]
- Takagi, Y.; Higashi, N.; Kawai, S.; Maeda, A.; Ueba, N. Effects of traditional oriental medicine on influenza virus infection: Enhancing effect of traditional oriental medicines on antibody production to B strain after vaccination with influenza HA vaccine in aged mice. Int. Congr. Ser. 2004, 1263, 532–535. [Google Scholar] [CrossRef]
- Kamei, T.; Kondoh, T.; Nagura, S.; Toriumi, Y.; Kumano, H.; Tomioka, H. Improvement of C-Reactive Protein Levels and Body Temperature of an Elderly Patient Infected with Pseudomonas aeruginosa on Treatment with Mao-Bushi-Saishin-To. J. Altern. Complement. Med. 2000, 6, 235–239. [Google Scholar] [CrossRef]
- Saito, S.-Y.; Kamiyama, S.; Oda, M.; Nakahata, N.; Ohizumi, Y. Inhibitory effect of Mao-Bushi-Saishin-to on prostaglandin E2 synthesis in C6 rat glioma cells. Biol. Pharm. Bull. 2004, 27, 1133–1135. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, T.; Tomioka, H.; Sato, K.; Sano, C.; Akaki, T.; Dekio, S.; Yamada, Y.; Kamei, T.; Shibata, H.; Higashi, N. Effects of the Chinese Traditional Medicine Mao-Bushi-Saishin-To on Therapeutic Efficacy of a New Benzoxazinorifamycin, KRM-1648, against Mycobacterium avium Infection in Mice. Antimicrob. Agents Chemother. 1999, 43, 514–519. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, T.; Sano, C.; Akaki, T.; Ogasawara, K.; Sato, K.; Tomioka, H. Effects of the Chinese traditional medicines “mao-bushi-saishin-to” and “yokuinin” on the antimycobacterial activity of murine macrophages against Mycobacterium avium complex infection. Kekkaku Tuberc. 1999, 74, 661–666. [Google Scholar]
- Burns, J.; Zhao, L.; Taylor, E.W.; Spelman, K. The influence of traditional herbal formulas on cytokine activity. Toxicology 2010, 278, 140–159. [Google Scholar] [CrossRef] [PubMed]
- Hikino, H.; Konno, C.; Takata, H.; Tamada, M. Antiinflammatory principle of Ephedra herbs. Chem. Pharm. Bull. 1980, 28, 2900–2904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mei, F.; Xing, X.-F.; Tang, Q.-F.; Chen, F.-L.; Guo, Y.; Song, S.; Tan, X.-M.; Luo, J.-B. Antipyretic and anti-asthmatic activities of traditional Chinese herb-pairs, Ephedra and Gypsum. Chin. J. Integr. Med. 2014, 22, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Kushida, H.; Matsushita, S.; Oyama, Y.; Suda, T.; Watanabe, J.; Kase, Y.; Setou, M. Distribution Analysis via Mass Spectrometry Imaging of Ephedrine in the Lungs of Rats Orally Administered the Japanese Kampo Medicine Maoto. Sci. Rep. 2017, 7, 44098. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, F.; Ghorani, V.; Saadat, S.; Gholamnezhad, Z.; Boskabady, M.H. The Stimulatory Effects of Medicinal Plants on β2-adrenoceptors of Tracheal Smooth Muscle. Iran J. Allergy Asthma Immunol. 2019, 18, 12–26. [Google Scholar] [CrossRef] [Green Version]
- Mizuno, K.; Shibata, K.; Komatsu, R.; Omiya, Y.; Kase, Y.; Koizumi, S. An effective therapeutic approach for oxaliplatin-induced peripheral neuropathy using a combination therapy with goshajinkigan and bushi. Cancer Biol. Ther. 2016, 17, 1206–1212. [Google Scholar] [CrossRef] [Green Version]
- Tanimura, Y.; Yoshida, M.; Ishiuchi, K.; Ohsawa, M.; Makino, T. Neoline is the active ingredient of processed aconite root against murine peripheral neuropathic pain model, and its pharmacokinetics in rats. J. Ethnopharmacol. 2019, 241, 111859. [Google Scholar] [CrossRef]
- Ohbuchi, K.; Miyagi, C.; Suzuki, Y.; Mizuhara, Y.; Mizuno, K.; Omiya, Y.; Yamamoto, M.; Warabi, E.; Sudo, Y.; Yokoyama, A.; et al. Ignavine: A novel allosteric modulator of the μ opioid receptor. Sci. Rep. 2016, 6, 31748. [Google Scholar] [CrossRef] [Green Version]
- Han, A.-R.; Kim, H.J.; Shin, M.; Hong, M.; Kim, Y.S.; Bae, H. Constituents of Asarum sieboldii with Inhibitory Activity on Lipopolysaccharide (LPS)-Induced NO Production in BV-2 Microglial Cells. Chem. Biodivers. 2008, 5, 346–351. [Google Scholar] [CrossRef]
- Kim, S.-J.; Zhang, C.G.; Lim, J.T. Mechanism of anti-nociceptive effects of Asarum sieboldii Miq. radix: Potential role of bradykinin, histamine and opioid receptor-mediated pathways. J. Ethnopharmacol. 2003, 88, 5–9. [Google Scholar] [CrossRef]
- Lee, J.Y.; Moon, S.S.; Hwang, B.K. Isolation and antifungal activity of kakuol, a propiophenone derivative from Asarum sieboldii rhizome. Pest Manag. Sci. 2005, 61, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-H.; Seo, S.-J.; Hur, J.-M.; Lee, H.-S.; Lee, Y.-E.; You, Y.-O. Asarum sieboldii Extracts Attenuate Growth, Acid Production, Adhesion, and Water-Insoluble Glucan Synthesis ofStreptococcus mutans. J. Med. Food 2006, 9, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Nishi, A.; Ohbuchi, K.; Kushida, H.; Matsumoto, T.; Lee, K.; Kuroki, H.; Nabeshima, S.; Shimobori, C.; Komokata, N.; Kanno, H.; et al. Deconstructing the traditional Japanese medicine “Kampo”: Compounds, metabolites and pharmacological profile of maoto, a remedy for flu-like symptoms. NPJ Syst. Biol. Appl. 2017, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Futamura-Masuda, M.; Yokota-Honda, M.; Anraku, T.; Nakanishi, K.; Murata, K.; Shinada, T.; Matsuda, H. Effect of Asiasarum Root Extract and Its Constituents on Interleukin-1β-Stimulated Matrix Metalloproteinase-1 Secretion from Human Gingival Fibroblasts. Biol. Pharm. Bull. 2016, 39, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Terada, Y. Food Compounds Activating Thermosensitive TRP Channels in Asian Herbal and Medicinal Foods. J. Nutr. Sci. Vitaminol. 2015, 61. [Google Scholar] [CrossRef] [PubMed]
- Sawada, R.; Iwata, M.; Umezaki, M.; Usui, Y.; Kobayashi, T.; Kubono, T.; Hayashi, S.; Kadowaki, M.; Yamanishi, Y. KampoDB, database of predicted targets and functional annotations of natural medicines. Sci. Rep. 2018, 8, 11216. [Google Scholar] [CrossRef]
- Li, C.; Xu, F.; Xie, D.-M.; Jing, Y.; Shang, M.-Y.; Liu, G.; Wang, X.; Cai, S.-Q. Identification of Absorbed Constituents in the Rabbit Plasma and Cerebrospinal Fluid after Intranasal Administration of Asari Radix et Rhizoma by HS-SPME-GC-MS and HPLC-APCI-IT-TOF-MSn. Molecules 2014, 19, 4857–4879. [Google Scholar] [CrossRef] [Green Version]
- Kitagawa, I.; Kinjyo, J.; Kuwajima, H.; Sankawa, U.; Syoji, J.; Takito, M.; Tomoda, M.; Nishioka, I.; Nohara, T.; Yamagishi, T. Shoyakugaku, 8th ed.; Hirokawa Publishing Co.: Kyoto, Japan, 2011; pp. 242–244. [Google Scholar]
- Mudge, E.; Lopes-Lutz, D.; Brown, P.; Schieber, A. Analysis of Alkylamides in Echinacea Plant Materials and Dietary Supplements by Ultrafast Liquid Chromatography with Diode Array and Mass Spectrometric Detection. J. Agric. Food Chem. 2011, 59, 8086–8094. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, K.; Wang, S.; Han, Y. Simultaneous Determination of Two Epimeric Furofuran Lignans (Sesamin and Asarinin) of Asarum heterotropoides Extract in Rat Plasma by LC/MS/MS: Application to Pharmacokinetic Study. J. Chromatogr. Sci. 2013, 52, 793–798. [Google Scholar] [CrossRef] [Green Version]
- Sakaki, T.; Yasuda, K.; Nishikawa, M.; Ikushiro, S. Metabolism of Sesamin and Drug-Sesamin Interaction. J. Pharm. Soc. Jpn. 2018, 138, 357–363. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhu, W.; Liu, H.; Wu, G.; Song, M.; Yang, B.; Yang, D.; Wang, Q.; Kuang, H.-X. Simultaneous Determination of Aesculin, Aesculetin, Fraxetin, Fraxin and Polydatin in Beagle Dog Plasma by UPLC-ESI-MS/MS and Its Application in a Pharmacokinetic Study after Oral Administration Extracts of Ledum palustre L. Molecules 2018, 23, 2285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitagawa, H.; Munekage, M.; Matsumoto, T.; Sadakane, C.; Fukutake, M.; Aoki, K.; Watanabe, J.; Maemura, K.; Hattori, T.; Kase, Y.; et al. Pharmacokinetic Profiles of Active Ingredients and Its Metabolites Derived from Rikkunshito, a Ghrelin Enhancer, in Healthy Japanese Volunteers: A Cross-Over, Randomized Study. PLoS ONE 2015, 10, e0133159. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Matsubara, Y.; Mizuhara, Y.; Sekiguchi, K.; Koseki, J.; Tsuchiya, K.; Nishimura, H.; Watanabe, J.; Kaneko, A.; Maemura, K.; et al. Plasma Pharmacokinetics of Polyphenols in a Traditional Japanese Medicine, Jumihaidokuto, Which Suppresses Propionibacterium acnes-Induced Dermatitis in Rats. Molecules 2015, 20, 18031–18046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizuhara, Y.; Takizawa, Y.; Ishihara, K.; Asano, T.; Kushida, H.; Morota, T.; Kase, Y.; Takeda, S.; Aburada, M.; Nomura, M.; et al. The Influence of the Sennosides on Absorption of Glycyrrhetic Acid in Rats. Biol. Pharm. Bull. 2005, 28, 1897–1902. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Kong, X.; Zhang, T.; Ye, J.; Fang, Z.; Yang, X. Pseudoephedrine/ephedrine shows potent anti-inflammatory activity against TNF-α-mediated acute liver failure induced by lipopolysaccharide/d-galactosamine. Eur. J. Pharmacol. 2014, 724, 112–121. [Google Scholar] [CrossRef]
- Bernatoniené, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, Y. The regulatory role of nitric oxide in proinflammatory cytokine expression during the induction and resolution of inflammation. J. Leukoc. Biol. 2010, 88, 1157–1162. [Google Scholar] [CrossRef]
- Romano, B.; Borrelli, F.; Fasolino, I.; Capasso, R.; Piscitelli, F.; Cascio, M.; Pertwee, R.G.; Coppola, D.; Vassallo, L.; Orlando, P.; et al. The cannabinoid TRPA1 agonist cannabichromene inhibits nitric oxide production in macrophages and ameliorates murine colitis. Br. J. Pharmacol. 2013, 169, 213–229. [Google Scholar] [CrossRef]
- Sahoo, S.S.; Majhi, R.K.; Tiwari, A.; Acharya, T.; Kumar, P.S.; Saha, S.; Kumar, A.; Goswami, C.; Chattopadhyay, S. Transient receptor potential ankyrin1 channel is endogenously expressed in T cells and is involved in immune functions. Biosci. Rep. 2019, 39, 39. [Google Scholar] [CrossRef] [Green Version]
- Kwan, K.; Corey, D.P. Burning Cold: Involvement of TRPA1 in Noxious Cold Sensation. J. Gen. Physiol. 2009, 133, 251–256. [Google Scholar] [CrossRef] [Green Version]
- Bandell, M.; Story, G.M.; Hwang, S.W.; Viswanath, V.; Eid, S.R.; Petrus, M.J.; Earley, T.J.; Patapoutian, A. Noxious Cold Ion Channel TRPA1 Is Activated by Pungent Compounds and Bradykinin. Neuron 2004, 41, 849–857. [Google Scholar] [CrossRef] [Green Version]
- Aubdool, A.A.; Kodji, X.; Abdul-Kader, N.; Heads, R.; Fernandes, E.S.; Bevan, S.; Brain, S.D. TRPA1 activation leads to neurogenic vasodilatation: Involvement of reactive oxygen nitrogen species in addition to CGRP and NO. Br. J. Pharmacol. 2016, 173, 2419–2433. [Google Scholar] [CrossRef] [Green Version]
- Masamoto, Y.; Kawabata, F.; Fushiki, T. Intragastric Administration of TRPV1, TRPV3, TRPM8, and TRPA1 Agonists Modulates Autonomic Thermoregulation in Different Manners in Mice. Biosci. Biotechnol. Biochem. 2009, 73, 1021–1027. [Google Scholar] [CrossRef]
- Liu, X.; Li, H.; Song, X.; Qin, K.; Guo, H.; Wu, L.; Cai, H.; Cai, B. Comparative pharmacokinetics studies of benzoylhypaconine, benzoylmesaconine, benzoylaconine and hypaconitine in rats by LC-MS method after administration of Radix Aconiti Lateralis Praeparata extract and Dahuang Fuzi Decoction. Biomed. Chromatogr. 2013, 28, 966–973. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, Q.; Wang, B.; Zhang, M.; Xu, Y.; Li, S.; Zhao, Y.; Yu, Z. Plasma Pharmacokinetics, Bioavailability, and Tissue Distribution of Four C-Glycosyl Flavones from Mung Bean (Vigna radiata L.) Seed Extracts in Rat by Ultrahigh-Performance Liquid Chromatography–Tandem Mass Spectrometry. J. Agric. Food Chem. 2017, 65, 5570–5580. [Google Scholar] [CrossRef] [PubMed]
- Jabor, V.A.P.; Soares, D.M.; Diniz, A.; De Souza, G.E.P.; Lopes, N.P. LC-MS-MS identification and determination of the flavone-C-glucoside vicenin-2 in rat plasma samples following intraperitoneal administration of Lychnophora extract. Nat. Prod. Commun. 2010, 5, 741–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsubara, Y.; Matsumoto, T.; Sekiguchi, K.; Koseki, J.; Kaneko, A.; Yamaguchi, T.; Kurihara, Y.; Kobayashi, H. Oral Administration of the Japanese Traditional Medicine Keishibukuryogan-ka-yokuinin Decreases Reactive Oxygen Metabolites in Rat Plasma: Identification of Chemical Constituents Contributing to Antioxidant Activity. Molecules 2017, 22, 256. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-J.; Maliakal, P.; Chen, L.; Meng, X.; Bondoc, F.Y.; Prabhu, S.; Lambert, G.; Mohr, S.; Yang, C.S. Pharmacokinetics of tea catechins after ingestion of green tea and (-)-epigallocatechin-3-gallate by humans: Formation of different metabolites and individual variability. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1025–1032. [Google Scholar]
- Ikeda, Y.; Oyama, T.; Taki, M.; Suzuki, Y.; Noguchi, M.; Omiya, Y.; Goto, K.; Komatsu, Y. Anti-nociceptive effect of mao-bushi-saishin-to in mice and rats. J. Trad. Med. 1996, 13, 81–86. [Google Scholar]
- Ohbuchi, K.; Mori, Y.; Ogawa, K.; Warabi, E.; Yamamoto, M.; Hirokawa, T. Detailed Analysis of the Binding Mode of Vanilloids to Transient Receptor Potential Vanilloid Type I (TRPV1) by a Mutational and Computational Study. PLoS ONE 2016, 11, e0162543. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |


| tR (min) | Adduct | Observed Intact Mass (Da) | Error (ppm) | Molecular Formula | Assigned and Estimated Compound from Our Chemical Database | AR-Treated Rat Plasma † | MBST-Treated Rat Plasma † | Characteristic Fragment Ions | Identification Results |
|---|---|---|---|---|---|---|---|---|---|
| 6.87 | +H | 148.0884 | −2.7 | C10H12O | Estragole | + | ND | ND | NI |
| 9.36 | +H | 281.2001 | 3.5 | C16H27NO3 | (2E,4E,9E)-8,11-Dihydroxy-N-isobutyl-2,4,9-dodecatrienamide, (2E,4E,8Z)-10,11-Dihydroxy-N-isobutyl-2,4,8-dodecatrienamide | + | + + + | ND | NI |
| 9.64 | +H | 281.2000 | 3.2 | C16H27NO3 | (2E,4E,9E)-8,11-Dihydroxy-N-isobutyl-2,4,9-dodecatrienamide, (2E,4E,8Z)-10,11-Dihydroxy-N-isobutyl-2,4,8-dodecatrienamide | + | + + + | 264.1963 | NI |
| 9.82 | +H | 281.1997 | 2.0 | C16H27NO3 | (2E,4E,9E)-8,11-Dihydroxy-N-isobutyl-2,4,9-dodecatrienamide, (2E,4E,8Z)-10,11-Dihydroxy-N-isobutyl-2,4,8-dodecatrienamide | + | + + + | ND | NI |
| 11.32 | −H | 357.0468 | −4.7 | C17H11NO8 | Aristolochic acid Iva | + | + + + | 310.0463, 307.0452, 281.0461, 266.0571, 265.0475, 251.0328 | NI |
| 11.49 | −H | 342.1094 | −2.8 | C19H18O6 | (7α,7′β,8α,8′α)-3,4-Methylenedioxy-3′,4′-dihydroxy-7,9′:7′,9-diepoxylignane | + | ND | 311.0910, 176.0458, 175.0396 | NI |
| 12.43 | +H | 208.0734 | −0.6 | C11H12O4 | Methyl kakuol | + | + + + | 191.0701, 179.0333, 176.0460, 165.0173 | Methyl kakuol |
| 15.05 | +H | 221.1780 | 0.3 | C14H23NO | Spilanthol | + | + + + | ND | NI |
| 16.30 | +H, +Na | 247.1937 | 0.4 | C16H25NO | Amide A | + | + + + | 167.1289, 166.1222 | Amide B # |
| 16.39 | +H, +Na | 247.1937 | 0.2 | C16H25NO | Amide A | + | + + + | 167.1298, 166.1221, 152.1040 | Amide A |
| 16.59 | +H | 247.1935 | −0.5 | C16H25NO | Amide A | + | + + + | ND | N-Isobutyl-2,4,8,10-dodecatetraenamide isomer # |
| Compound | Dose of MBST (g/kg Body Weight) | Dose of Ingredient within MBST (µg/kg) | Cmax (ng/mL) | tmax (h) | AUC0-last (ng·h/mL) | t1/2 (h) | kel (h−1) |
|---|---|---|---|---|---|---|---|
| Asarinin | 1 | 578 | 8.42 | 0.25 | 16.3 | 1.52 | 0.456 |
| 2 | 1156 | 14.7 | 0.25 | 38.6 | 2.93 | 0.237 | |
| Sesamin | 1 | 147 | 5.63 | 0.5 | 11.9 | 1.63 | 0.425 |
| 2 | 294 | 9.44 | 0.25 | 28.7 | 2.99 | 0.232 | |
| Methyl kakuol | 1 | 226 | 26.7 | 0.5 | 22.5 | 0.452 | 1.53 |
| 2 | 452 | 98.2 | 1.0 | 214 | 1.26 | 0.552 | |
| Amide A | 1 | 426 | 4.08 | 0.5 | 4.87 | 0.912 | 0.760 |
| 2 | 852 | 6.07 | 0.25 | 18.4 | 2.92 | 0.237 |
| Crude Drug | Test Compound | % of Activation Compared to Positive Control against Each Subfamily of TRP Channel | EC50 Value against TRPA1 (µmol/L) | Pharmacokinetics Study Reference | |||
|---|---|---|---|---|---|---|---|
| TRPA1 | TRPV1 | TRPV4 | TRPM8 | ||||
| Asiasari Radix | Methyl kakuol | 49 | 0.1 | 0.9 | 2.8 | 0.27 | Herein |
| Amide A | 96 | 0.7 | 2.7 | 6.1 | 0.47 | Herein | |
| Asarinin | 106 | −0.4 | −0.1 | 1.4 | 3.1 | Herein | |
| Sesamin | 106 | −0.1 | −0.1 | −0.8 | 2.3 | Herein | |
| Aconiti Radix Processa | Neoline | 5 | −0.4 | 0.5 | 5.0 | — | [27] |
| Ignavine | 2 | −0.2 | −0.1 | 1.8 | — | [28] | |
| Benzoylaconitine | 3 | −0.2 | 0.0 | 3.6 | — | [55] | |
| Ephedrae Herba | Isovitexin | 18 | 0.2 | 2.3 | 4.3 | — | [56] |
| Vicenin-2 | 25 | 1.8 | 5.0 | 6.5 | — | [57] | |
| Ephedrine | 3 | −0.5 | −0.4 | 3.2 | — | [33] | |
| Pseudoephedrine | 4 | −0.2 | −0.3 | 2.8 | — | [33] | |
| Methylephedrine | 3 | −0.4 | −0.1 | 3.6 | — | [33] | |
| Methylephedrine N-oxide | 21 | 0.3 | 1.8 | 4.5 | — | [33] | |
| Catechin | 6 | −0.3 | 0.3 | 4.0 | — | [58] | |
| Epicatechin | 6 | 0.0 | 0.6 | 4.2 | — | [59] | |
| Epigallocatechin | 2 | −0.2 | 0.1 | 4.8 | — | [59] | |
| Hippuric acid | 13 | 1.0 | 2.8 | 7.5 | — | [33] | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumoto, T.; Takiyama, M.; Sanechika, S.; Nakayama, A.; Aoki, K.; Ohbuchi, K.; Kushida, H.; Kanno, H.; Nishi, A.; Watanabe, J. In Vivo Pharmacokinetic Analysis Utilizing Non-Targeted and Targeted Mass Spectrometry and In Vitro Assay against Transient Receptor Potential Channels of Maobushisaishinto and Its Constituent Asiasari Radix. Molecules 2020, 25, 4283. https://doi.org/10.3390/molecules25184283
Matsumoto T, Takiyama M, Sanechika S, Nakayama A, Aoki K, Ohbuchi K, Kushida H, Kanno H, Nishi A, Watanabe J. In Vivo Pharmacokinetic Analysis Utilizing Non-Targeted and Targeted Mass Spectrometry and In Vitro Assay against Transient Receptor Potential Channels of Maobushisaishinto and Its Constituent Asiasari Radix. Molecules. 2020; 25(18):4283. https://doi.org/10.3390/molecules25184283
Chicago/Turabian StyleMatsumoto, Takashi, Mikina Takiyama, Shou Sanechika, Akiko Nakayama, Katsuyuki Aoki, Katsuya Ohbuchi, Hirotaka Kushida, Hitomi Kanno, Akinori Nishi, and Junko Watanabe. 2020. "In Vivo Pharmacokinetic Analysis Utilizing Non-Targeted and Targeted Mass Spectrometry and In Vitro Assay against Transient Receptor Potential Channels of Maobushisaishinto and Its Constituent Asiasari Radix" Molecules 25, no. 18: 4283. https://doi.org/10.3390/molecules25184283