Structure and Physical Properties of Cardamonin: A Spectroscopic and Computational Approach
Abstract
:1. Introduction
2. Results and Discussion
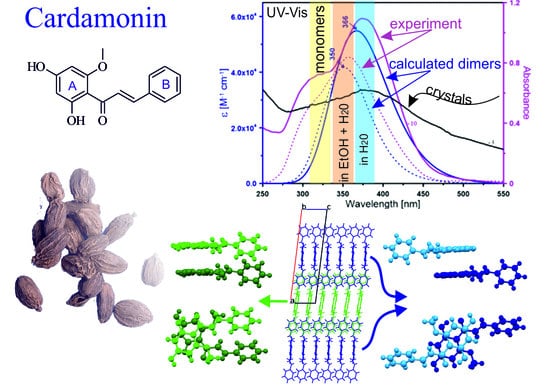
2.1. Crystal Structure Description
2.2. Theoretical Calculations
2.3. Spectral Properties
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. UV-Vis Spectroscopy
3.2.2. Steady-State Fluorescence Spectroscopy
3.2.3. Single Crystal X-ray Diffraction
3.2.4. Gaussian16 Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tuan, H.N.; Minh, B.H.; Tran, P.T.; Lee, J.H.; Oanh, H.V.; Ngo, Q.M.T.; Nguyen, Y.N.; Lien, P.T.K.; Tran, M.H. The Effects of 2′,4′-Dihydroxy-6′-methoxy-3′,5′- dimethylchalcone from Cleistocalyx operculatus Buds on Human Pancreatic Cancer Cell Lines. Molecules 2019, 24, 2538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu Bakar, A.; Akhtar, M.N.; Ali, N.M.; Yeap, S.K.; Quah, C.K.; Loh, W.S.; Alitheen, N.B.; Zareen, S.; Ul-Haq, Z.; Shah, S.A.A. Design, synthesis and docking studies of flavokawain B type chalcones and their cytotoxic effects on MCF-7 and MDA-MB-231 cell lines. Molecules 2018, 23, 616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budziak, I.; Arczewska, M.; Kaminski, D.M. Formation of prenylated chalcone xanthohumol cocrystals: Single crystal X-ray diffraction, vibrational spectroscopic study coupled with multivariate analysis. Molecules 2019, 24, 4245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogel, S.; Ohmayer, S.; Brunner, G.; Heilmann, J. Natural and non-natural prenylated chalcones: Synthesis, cytotoxicity and anti-oxidative activity. Bioorgan. Med. Chem. 2008, 16, 4286–4293. [Google Scholar] [CrossRef]
- Gaonkar, S.L.; Vignesh, U.N. Synthesis and pharmacological properties of chalcones: A review. Res. Chem. Intermediat. 2017, 43, 6043–6077. [Google Scholar] [CrossRef]
- Carvalho, P.S., Jr.; Custodio, J.M.; Vaz, W.F.; Cirilo, C.C.; Cidade, A.F.; Aquino, G.L.; Campos, D.M.; Cravo, P.; Coelho, C.J.; Oliveira, S.S.; et al. Conformation analysis of a novel fluorinated chalcone. J. Mol. Model. 2017, 23, 97. [Google Scholar] [CrossRef]
- Ekbote, A.; Patil, P.S.; Maidur, S.R.; Chia, T.S.; Quah, C.K. Structural, third-order optical nonlinearities and figures of merit of(E)-1-(3-substituted phenyl)-3-(4-fluorophenyl) prop-2-en-1-oneunder CW regime: New chalcone derivatives for optical limiting applications. Dyes Pigm. 2017, 139, 720–729. [Google Scholar] [CrossRef]
- Jaiswal, S.; Sharma, A.; Shukla, M.; Lal, J. Gender-related pharmacokinetics and bioavailability of a novel anticancer chalcone, cardamonin, in rats determined by liquid chromatography tandem mass spectrometry. J. Chromatogr. B 2015, 986, 23–30. [Google Scholar] [CrossRef]
- Zhang, J.W.; Sikka, S.; Siveen, K.S.; Lee, J.H.; Um, J.Y.; Kumar, A.P.; Chinnathambi, A.; Alharbi, S.A.; Basappa; Rangappa, K.S.; et al. Cardamonin represses proliferation, invasion, and causes apoptosis through the modulation of signal transducer and activator of transcription 3 pathway in prostate cancer. Apoptosis 2017, 22, 158–168. [Google Scholar] [CrossRef]
- Shrivastava, S.; Jeengar, M.K.; Thummuri, D.; Koval, A.; Katanaev, V.L.; Marepally, S.; Naidu, V.G.M. Cardamonin, a chalcone, inhibits human triple negative breast cancer cell invasiveness by downregulation of Wnt/-beta-catenin signaling cascades and reversal of epithelial-mesenchymal transition. Biofactors 2017, 43, 152–169. [Google Scholar] [CrossRef] [Green Version]
- James, S.; Aparna, J.S.; Paul, A.M.; Lankadasari, M.B.; Mohammed, S.; Binu, V.S.; Santhoshkumar, T.R.; Reshmi, G.; Harikumar, K.B. Cardamonin inhibits colonic neoplasia through modulation of MicroRNA expression. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Park, S.; Gwak, J.; Han, S.J.; Oh, S. Cardamonin suppresses the proliferation of colon cancer cells by promoting beta-catenin degradation. Biol. Pharm. Bull. 2013, 36, 1040–1044. [Google Scholar] [CrossRef] [Green Version]
- Ping, C.P.; Mohamad, T.A.S.T.; Akhtar, M.N.; Perimal, E.K.; Akira, A.; Ali, D.A.I.; Sulaiman, M.R. Antinociceptive effects of cardamonin in mice: Possible involvement of TRPV1, glutamate, and opioid receptors. Molecules 2018, 23, 2237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, S.J.; Hou, Y.N.; Yao, J.; Fang, J.G. Activation of Nrf2-driven antioxidant enzymes by cardamonin confers neuroprotection of PC12 cells against oxidative damage. Food Funct. 2017, 8, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Gong, X. The conformational, electronic and spectral properties of chalcones: A density functional theory study. J. Mol. Struct. 2009, 901, 226–231. [Google Scholar] [CrossRef]
- Arczewska, M.; Kaminski, D.M.; Gieroba, B.; Gagos, M. Acid-base properties of xanthohumol: A computational and experimental investigation. J. Nat. Prod. 2017, 80, 3195–3203. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Wu, J.; Liu, X.; Zhao, B.; Wang, Z. Study on structural and spectral properties of isobavachalcone and 4-hydroxyderricin by computational method. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 126, 254–259. [Google Scholar] [CrossRef]
- Memon, A.H.; Ismail, Z.; Aisha, A.F.; Al-Suede, F.S.; Hamil, M.S.; Hashim, S.; Saeed, M.A.; Laghari, M.; Abdul Majid, A.M. Isolation, characterization, crystal Structure elucidation, and anticancer study of dimethyl cardamonin, isolated from Syzygium campanulatum Korth. Evid. Based Complement. Alternat. Med. 2014, 2014, 470179. [Google Scholar] [CrossRef] [Green Version]
- Karabacak, M.; Kose, E.; Atac, A.; Asiri, A.M.; Kurt, M. Monomeric and dimeric structures analysis and spectroscopic characterization of 3,5-difluorophenylboronic acid with experimental (FT-IR, FT-Raman, H-1 and C-13 NMR, UV) techniques and quantum chemical calculations. J. Mol. Struct. 2014, 1058, 79–96. [Google Scholar] [CrossRef]
- Olsztynska-Janus, S.; Szymborska, K.; Komorowska, M.; Lipinski, J. Conformational changes of L-phenylalanine - Near infrared-induced mechanism of dimerization: B3LYP studies. J. Mol. Struct. 2009, 911, 1–7. [Google Scholar] [CrossRef]
- Shukla, S.; Srivastava, A.; Srivastava, K.; Tandon, P.; Jamalis, J.; Singh, R.B. Non-covalent interactions and spectroscopic study of chalcone derivative 1-(4-chlorophenyl)-3-(5-methylfuran-2-yl) prop-2-en-1-one. J. Mol. Struct. 2020, 1201. [Google Scholar] [CrossRef]
- Custodio, J.M.F.; Guimaraes-Neto, J.J.A.; Awad, R.; Queiroz, J.E.; Verde, G.M.V.; Mottin, M.; Neves, B.J.; Andrade, C.H.; Aquino, G.L.B.; Valverde, C.; et al. Molecular modelling and optical properties of a novel fluorinated chalcone. Arab. J. Chem. 2020, 13, 3362–3371. [Google Scholar] [CrossRef]
- Zhuang, C.; Zhang, W.; Sheng, C.; Xing, C.; Miao, Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef] [PubMed]
- Aljancic, I.S.; Vuckovic, I.; Jadranin, M.; Pesic, M.; Dordevic, I.; Podolski-Renic, A.; Stojkovic, S.; Menkovic, N.; Vajs, V.E.; Milosavljevic, S.M. Two structurally distinct chalcone dimers from Helichrysum zivojinii and their activities in cancer cell lines. Phytochemistry 2014, 98, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Menezes, C.J.M.D.S.; Diederich, M.F. Natural dimers of coumarin, chalcones, and resveratrol and the link between structure and pharmacology. Eur. J. Med. Chem. 2019, 182, 111637. [Google Scholar] [CrossRef]
- Tauc, J. The Optical Properties of Solids; Academic Press: New York, NY, USA, 1966. [Google Scholar]
- Zhou, Z.X.; Parr, R.G. Activation hardness—New index for describing the orientation of electrophilic aromatic-substitution. J. Am. Chem. Soc. 1990, 112, 5720–5724. [Google Scholar] [CrossRef]
- Kaminski, D.M.; Gaweda, K.; Arczewska, M.; Senczyna, B.; Gagos, M. A kinetic study of xanthohumol cyclization to isoxanthohumol - A role of water. J. Mol. Struct. 2017, 1139, 10–16. [Google Scholar] [CrossRef]
- Maidur, S.R.; Jahagirdar, J.R.; Patil, P.S.; Chia, T.S.; Quah, C.K. Structural characterizations, Hirshfeld surface analyses, and third order nonlinear optical properties of two novel chalcone derivatives. Opt. Mater. 2018, 75, 580–594. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Gao, J.; Lu, R.; Liu, F. Adjustment of the solid fluorescence of a chalcone derivative through controlling steric hindrance. RSC Adv. 2017, 7, 46354–46357. [Google Scholar] [CrossRef] [Green Version]
- Karuppusamy, A.; Vandana, T.; Kannan, P. Pyrene based chalcone materials as solid state luminogens with aggregation-induced enhanced emission properties. J. Photochem. Photobiol. A. 2017, 345, 11–20. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Liu, L.; Luo, H.; Wang, H.; Yang, D.; He, F. Flavanone-based fluorophores with aggregation-induced emission enhancement characteristics for mitochondria-imaging and zebrafish-imaging. Molecules 2020, 25, 3298. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Liu, B. Aggregation-induced emission: Recent advances in materials and biomedical applications. Angew. Chem. Int. Ed. 2020, 59, 9868–9886. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, F.; Zhang, H.; Xie, Z.; Xie, W.; Xu, H.; Li, B.; Shen, F.; Ye, L.; Hanif, M.; et al. Tight intermolecular packing through supramolecular interactions in crystals of cyano substituted oligo (para-phenylenevinylene): A key factor for aggregation-induced emission. ChemComm 2007, 23, 1–3. [Google Scholar]
- Cai, M.; Gao, Z.; Zhou, X.; Wang, X.; Chen, S.; Zhao, Y.; Qian, Y.; Shi, N.; Mi, B.; Xie, L.; et al. A small change in molecular structure, a big difference in the AIEE mechanism. Phys. Chem. Chem. Phys. 2012, 14, 5289–5296. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Adamo, C.; Jacquemin, D. The calculations of excited-state properties with Time-Dependent Density Functional Theory. Chem. Soc. Rev. 2013, 42, 845–856. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |







| Molecular Formula | C20H20O3 |
|---|---|
| Temperature (K) | 293(2) |
| Crystal system | monoclinic |
| Space group | P21/c |
| a (Å) | 25.7114(6) |
| b (Å) | 15.3506(3) |
| c (Å) | 6.8717(2) |
| α (°) | 90 |
| β (°) | 97.058(3) |
| γ (°) | 90 |
| V (Å3) | 2691.60(12) |
| Z | 8 |
| Calculated density (g cm−3) | 1.3339 |
| Absorption coefficient (mm−1) | 0.792 |
| F(000) | 1140 |
| Completeness | 99% |
| θ range for data collection (°) | 4.499–68.185 |
| Index ranges | −26 ≤ h ≤ 30 |
| −17 ≤ k ≤ 18 | |
| −7 ≤ l ≤ 8 | |
| Reflections collected/unique | 25,350/4917 |
| (Rint = 0.0355) | |
| Observed/restraints/parameters | 2073/0/368 |
| Goodness of fit on F2 | 1.0594 |
| Final R indices (I > 2sigma(I)) | R1 = 0.0678 |
| wR2 = 0.2274 | |
| R indices (all data) | R1 = 0.0839 |
| wR2 = 0.2409 | |
| Largest diff. peak and hole (e Å−3) | 0.3/−0.3 |
| CCDC Number | 2014912 |
| D–H···A | D–H (Å) | H···A (Å) | Angle (degree) | Symmetry |
|---|---|---|---|---|
| O1A–H2OA···O2A | 0.82 | 1.761 | 148.3 | x, y, z |
| O1A–H3OA···O3A | 0.82 | 1.907 | 173.9 | 2 − x, −1/2 + y, 1.5 − z |
| O1B···H2OB–O2B | 0.82 | 1.774 | 146.7 | x, y, z |
| O3B–H3OB···O3B | 0.82 | 1.898 | 174.7 | 1 − x, −1/2 + y, 1.5−z |
| Dimer | Energy (a.u.) |
|---|---|
| II | 0 |
| III | 8.56 |
| I | 26.85 |
| VII | 28.88 |
| V | 31.98 |
| IV | 72.07 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budziak, I.; Arczewska, M.; Kamiński, D.M. Structure and Physical Properties of Cardamonin: A Spectroscopic and Computational Approach. Molecules 2020, 25, 4070. https://doi.org/10.3390/molecules25184070
Budziak I, Arczewska M, Kamiński DM. Structure and Physical Properties of Cardamonin: A Spectroscopic and Computational Approach. Molecules. 2020; 25(18):4070. https://doi.org/10.3390/molecules25184070
Chicago/Turabian StyleBudziak, Iwona, Marta Arczewska, and Daniel M. Kamiński. 2020. "Structure and Physical Properties of Cardamonin: A Spectroscopic and Computational Approach" Molecules 25, no. 18: 4070. https://doi.org/10.3390/molecules25184070