Coupling Ultrafiltration-Based Processes to Concentrate Phenolic Compounds from Aqueous Goji Berry Extracts
Abstract
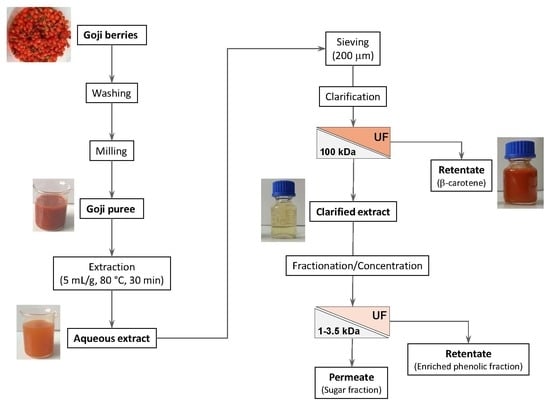
:1. Introduction
2. Results and Discussion
2.1. Clarification of Aqueous Extract with Hollow Fiber Membranes
2.2. Influence of Molecular Weight Cut-off and Transmembrane Pressure on the Performance of UF Membranes
2.3. Experiments in Diafiltration and Concentration Mode with GH Membrane
3. Material and Methods
3.1. Aqueous Extract from Goji Berries
3.2. Clarification of Aqueous Extract: Equipment and Procedures
3.3. Treatment of Clarified Extract with Tight UF Membranes: Equipment and Procedures
3.3.1. Screening Tests
3.3.2. Experiments with the GH Membrane in Diafiltration and Concentration Mode
3.4. Chemical Analysis and Determinations
3.4.1. Total Dissolved Solids (TDS) and Total Suspended Solids (TSS)
3.4.2. β-Carotene
3.4.3. Total Phenolic Content
3.4.4. In Vitro Antioxidant Activity
3.4.5. Total Carbohydrates
4. Conclusions
- a retentate fraction from the clarification process that could be considered a new source of β-carotene for different food applications (i.e., as natural, nontoxic food colorants or food supplements);
- a retentate fraction from the diafiltration/concentration process with a GH membrane enriched in polyphenols with high antioxidant capacity of interest in the food, pharmaceutical and cosmetic industries;
- a permeate fraction from the diafiltration/concentration process with a GH membrane enriched in sugars of interest for applications in the food industry.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Protti, M.; Gualandi, I.; Mandrioli, R.; Zappoli, S.; Tonelli, D.; Mercolini, L. Analytical profiling of selected antioxidants and total antioxidant capacity of goji (Lycium spp.) berries. J. Pharm. Biomed. Anal. 2017, 143, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Kulczyński, B.; Gramza-Michałowska, A. Goji Berry (Lycium barbarum): Composition and health effects—A review. Pol. J. Food Nutr. Sci. 2016, 66, 67–75. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic compounds profile, nutritional compounds and bioactive properties of Lycium barbarum L.: A comparative study with stems and fruits. Ind. Crops Prod. 2018, 122, 574–581. [Google Scholar] [CrossRef] [Green Version]
- Mocan, A.; Cairone, F.; Locatelli, M.; Cacciagrano, F.; Carradori, S.; Vodnar, D.C.; Cris, G.; Simonetti, G.; Cesa, S. Polyphenols from Lycium barbarum (Goji) fruit European cultivars at different maturation steps: Extraction, HPLC-DAD analyses, and biological evaluation. Antioxidants 2019, 8, 562. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Wei, Y.; Wang, Y.; Gao, F.; Chen, Z. Lycium Barbarum: A traditional Chinese herb and a promising anti-aging agent. Aging Dis. 2017, 8, 778–791. [Google Scholar] [CrossRef] [Green Version]
- Amagase, H.; Farnsworth, N.R. A review of botanical characteristics, phytochemistry, clinical relevance in efficacy and safety of Lycium barbarum fruit (Goji). Food Res. Int. 2011, 44, 1702–1717. [Google Scholar] [CrossRef]
- Inbaraj, B.S.; Lu, H.; Kao, T.H.; Chen, B.H. Simultaneous determination of phenolic acids and flavonoids in Lycium barbarum Linnaeus by HPLC–DAD–ESI-MS. J. Pharm. Biomed. Anal. 2010, 51, 549–556. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, W.; Zhao, J.; Xi, W. Functional constituents and antioxidant activities of eight Chinese native goji genotypes. Food Chem. 2016, 200, 230–236. [Google Scholar] [CrossRef]
- Donno, D.; Beccaro, G.L.; Mellano, M.G.; Cerutti, A.K.; Bounous, G. Goji berry fruit (Lycium spp.): Antioxidant compound fingerprint and bioactivity evaluation. J. Funct. Food. 2015, 18, 1070–1085. [Google Scholar] [CrossRef]
- Yao, R.; Heinrich, M.; Weckerle, C.S. The genus Lycium as food and medicine: A botanical, ethnobotanical and historical review. J. Ethnopharmacol. 2018, 212, 50–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Z.F.; Zhang, H.; Teh, S.S.; Wang, C.W.; Zhang, Y.; Hayford, F.; Wang, L.; Ma, T.; Dong, Z.; Zhang, Y.; et al. Goji berries as a potential natural antioxidant medicine: An insight into their molecular mechanisms of action. Oxid. Med. Cell. Longev. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.C.; Chang, S.C.; Inbaraj, B.S.; Chen, B.H. Isolation of carotenoids, flavonoids and polysaccharides from Lycium barbarum L. and evaluation of antioxidant activity. Food Chem. 2010, 120, 184–192. [Google Scholar] [CrossRef]
- Inbaraj, B.S.; Lu, H.; Hung, C.F.; Wu, W.B.; Lin, C.L.; Chen, B.H. Determination of carotenoids and their esters in fruits of Lycium barbarum Linnaeus by HPLC-DAD-APCI-MS. J. Pharm. Biomed. Anal. 2008, 47, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Fratianni, A.; Niro, S.; Alam, M.D.R.; Cinquanta, L.; Di Matteo, M.; Adiletta, G.; Panfili, G. Effect of a physical pre-treatment and drying on carotenoids of goji berries (Lycium barbarum L.). LWT-Food Sci. Technol. 2018, 92, 318–332. [Google Scholar] [CrossRef]
- Gil-Chávez, G.J.; Villa, J.A.; Ayala-Zavala, J.F.; Heredia, J.B.; Sepulveda, D.; Yahia, E.M.; González-Aguilar, G.A. Technologies for extraction and production of bioactive compounds to be used as nutraceuticals and food ingredients: An overview. Compr. Rev. Food. Sci. Food Saf. 2013, 12, 5–23. [Google Scholar] [CrossRef]
- Shi, J.; Mittal, G.; Kim, E.; Xue, J.S. Solubility of carotenoids in supercritical CO2. Food Rev. Int. 2007, 23, 341–371. [Google Scholar] [CrossRef]
- Miron, T.; Plaza, M.; Bahrim, G.; Ibáñez, E.; Herrero, M. Chemical composition of bioactive pressurized extracts of Romanian aromatic plants. J. Chromatogr. 2010, 1218, 4918–4927. [Google Scholar] [CrossRef] [Green Version]
- Herrero, M.; Cifuentes, A.; Ibanez, E. Sub and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae: A review. Food Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef] [Green Version]
- Esclapez, M.; García-Pérez, J.; Mulet, A.; Cárcel, J. Ultrasound-assisted extraction of natural products. Food Eng. Rev. 2011, 3, 108–120. [Google Scholar] [CrossRef]
- Zhang, H.F.; Yang, X.H.; Wang, Y. Microwave assisted extraction of secondary metabolites from plants: Current status and future directions. Trends Food Sci. Technol. 2011, 22, 672–688. [Google Scholar] [CrossRef]
- Raks, V.; Al-Suod, H.; Buszewsk, B. Isolation, separation, and preconcentration of biologically active compounds from plant matrices by extraction techniques. Chromatographia 2018, 81, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, L.; Dhuique-Mayer, C.; Castillo, S.; Vaillant, F.; Servent, A.; Dornier, M. Concentration and purification of lycopene from watermelon juice by integrated microfiltration-based processes. Innov. Food Sci. Emerg. Technol. 2016, 37, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Cassano, A.; Bentivenga, A.; Conidi, C.; Galiano, F.; Saoncella, O.; Figoli, A. Membrane-based clarification and fractionation of red wine lees aqueous extracts. Polymers 2019, 11, 1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conidi, C.; Egea-Corbacho, A.; Cassano, A. A combination of aqueous extraction and polymeric membranes as a sustainable process for the recovery of polyphenols from olive mill solid wastes. Polymers 2019, 11, 1868. [Google Scholar] [CrossRef] [Green Version]
- Valencia-Arredondo, J.A.; Hernández-Bolio, G.I.; Cerón-Montes, G.I.; Castro-Muñoz, R.; Yáñez-Fernández, J. Enhanced process integration for the extraction, concentration and purification of di-acylated cyanidin from red cabbage. Sep. Purif. Technol. 2020, 238, 116492. [Google Scholar] [CrossRef]
- Polidori, J.; Dhuique-Mayer, C.; Dornier, M. Crossflow microfiltration coupled with diafiltration to concentrate and purify carotenoids and flavonoids from citrus juices. Innov. Food Sci. Emerg. Technol. 2018, 45, 320–329. [Google Scholar] [CrossRef]
- Galiano, F.; Figoli, A.; Conidi, C.; Menechini, F.; Bonesi, M.; Loizzo, M.R.; Cassano, A.; Tundis, R. Functional properties of Punica granatum L. juice clarified by hollow fiber membranes. Processes 2016, 4, 21. [Google Scholar] [CrossRef]
- De Carvalho, L.M.J.; de Castro, I.M.; da Silva, C.A.B. A study of retention of sugars in the process of clarification of pineapple juice (Ananas comosus, L. Merril) by micro- and ultra-filtration. J. Food Eng. 2008, 87, 447–454. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Bąbelewski, P. Phenolic and carotenoid profile of new goji cultivars and their antihyperglycemic, anti-aging and antioxidant properties. J. Funct. Food 2018, 48, 632–642. [Google Scholar] [CrossRef]
- El-Agamey, A.; Lowen, G.M.; McGarvey, D.J.; Mortensen, A.; Phillip, D.M.; Truscott, T.G.; Young, A.J. Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch. Biochem. Biophys. 2004, 430, 37–48. [Google Scholar] [CrossRef]
- Landete, J.M. Dietary intake of natural antioxidants: Vitamins and polyphenols. Crit. Rev. Food Sci. Nutr. 2013, 53, 706–721. [Google Scholar] [CrossRef] [PubMed]
- Wackerbarth, H.; Stoll, T.; Gebken, S.; Pelters, C.; Bindrich, U. Carotenoid-protein interaction as an approach for the formulation of functional food emulsions. Food Res. Int. 2009, 42, 1254–1258. [Google Scholar] [CrossRef]
- Razi, B.; Aroujalian, A.; Fathizadeh, M. Modeling of fouling layer deposition in cross-flow microfiltration during tomato juice clarification. Food Bioprod. Process. 2012, 90, 841–848. [Google Scholar] [CrossRef]
- Vaillant, F.; Cissé, M.; Chaverri, M.; Perez, A.; Dornier, M.; Viquez, F.; Dhuique-Mayer, C. Clarification and concentration of melon juice using membrane processes. Innov. Food Sci. Emerg. Technol. 2005, 6, 213–220. [Google Scholar] [CrossRef]
- Paes, J.; Cunha, C.R.; Viotto, L.V. Concentration of lycopene in the pulp of papaya (Carica papaya L.) by ultrafiltration on a pilot scale. Food Bioprod. Process. 2015, 96, 296–305. [Google Scholar] [CrossRef]
- Machado, M.T.C.; Mello, B.C.B.S.; Hubinger, M.D. Evaluation of pequi (Caryocar Brasiliense Camb.) aqueous extract quality processed by membranes. Food Bioprod. Process. 2015, 95, 304–312. [Google Scholar] [CrossRef]
- Rai, P.; Majumdar, G.C.; Das Gupta, S.; De, S. Effect of various pretreatment methods on permeate flux and quality during ultrafiltration of mosambi juice. J. Food Eng. 2007, 78, 561–568. [Google Scholar] [CrossRef]
- Conidi, C.; Cassano, A.; Caiazzo, F.; Drioli, E. Separation and purification of phenolic compounds from pomegranate juice by ultrafiltration and nanofiltration membranes. J. Food Eng. 2017, 195, 1–13. [Google Scholar] [CrossRef]
- Díaz-Reinoso, B.; Moure, A.; Domínguez, H.; Parajó, J.C. Ultra- and nanofiltration of aqueous extracts from distilled fermented grape pomace. J. Food Eng. 2009, 91, 587–593. [Google Scholar] [CrossRef]
- Benítez, J.; Acero, J.L.; Leal, A.I.; González, M. The use of ultrafiltration and nanofiltration membranes for the purification of cork processing wastewater. J. Hazard. Mater. 2009, 162, 1438–1445. [Google Scholar] [CrossRef]
- Acosta, O.; Vaillant, F.; Pérez, A.M.; Dornier, M. Potential of ultrafiltration for separation and purification of ellagitannins in blackberry (Rubus adenotrichus Schltdl.) juice. Sep. Purif. Technol. 2014, 125, 120–125. [Google Scholar] [CrossRef]
- Balyan, U.; Sarkar, B. Integrated membrane processes for purification and concentration of aqueous Syzygium cumini (L.) seed extract. Food Bioprod. Process. 2016, 98, 29–43. [Google Scholar] [CrossRef]
- Cissé, M.; Vaillant, F.; Pallet, D.; Dorier, M. Selecting ultrafiltration and nanofiltration membranes to concentrate anthocyanins from roselle extract (Hibiscus sabdariffa L.). Food Res. Int. 2011, 44, 2607–2614. [Google Scholar] [CrossRef]
- Schütte, T.; Niewersch, C.; Wintgens, T.; Yüce, S. Phosphorus recovery from sewage by nanofiltration in diafiltration mode. J. Membr. Sci. 2015, 480, 74–82. [Google Scholar] [CrossRef]
- Syed, U.T.; Brazinha, C.; Crespo, J.C.; Ricardo-da-Silva, J.M. Valorisation of grape pomace: Fractionation of bioactive flavan-3-ols by membrane processing. Sep. Purif. Technol. 2017, 172, 404–414. [Google Scholar] [CrossRef]
- Lime, B.J.; Griffiths, F.P.; O’Connor, R.T.; Heinzelman, D.C.; McCall, E.R. Spectrophotometric methods for determining pigmentation—Beta-carotene and lycopene—In ruby red grapefruit. J. Agric. Food Chem. 1957, 5, 941–944. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzimol. 1999, 299, 152–178. [Google Scholar]
- Rice-Evans, C.A.; Miller, N.J. Total antioxidant status in plasma and body fluids. Methods Enzymol. 1994, 234, 279–293. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C.A. Antioxidant activity applying and improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.; Hamilton, J.; Rebers, P.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |






| Parameters | PS Membranes | PVDF Membranes | ||||
|---|---|---|---|---|---|---|
| Feed | Permeate | Retentate | Feed | Permeate | Retentate | |
| TSS (%) | 1.6 ± 1.1 | n.d. | 5.8 ± 0.2 | 1.6 ± 0.2 | n.d. | 5.8 ± 0.1 |
| TDS (° Brix) | 4.5 ± 0.4 | 4.0 ± 0.2 | 4.5 ± 1.2 | 4.2 ± 0.1 | 4.1 ± 0.2 | 4.2 ± 0.05 |
| β-carotene (μg/mL) | 2.80 ± 0.5 | 0.26 ± 0.05 | 4.74 ± 0.25 | 3.16 ± 0.1 | 0.098 ± 0.02 | 4.34 ± 0.35 |
| TPC (mg GAE/L) | 520.5 ± 2.6 | 510.0 ± 6.0 | 540.0 ± 8.0 | 448.5 ± 2.6 | 420 ± 6.0 | 480 ± 6.0 |
| TAA (mM Trolox) | 3.5 ± 0.5 | 2.6 ± 0.2 | 3.4 ± 0.12 | 2.8 ± 0.3 | 2.05 ± 0.25 | 2.6 ± 0.4 |
| TC (g glucose/L) | 15.1 ± 2.3 | 14.35 ± 1.5 | 18.2 ± 2.3 | 17.7 ± 3.2 | 17.5 ± 2.6 | 18.67 ± 2.4 |
| Membrane Type | |||
|---|---|---|---|
| GK | GH | GE | |
| Wp0 (L/m2hbar) | 9.26 | 4.19 | 3.28 |
| Wp1 (L/m2hbar) | 8.62 | 4.08 | 1.84 |
| Wp2 (L/m2hbar) | 9.104 | 2.62 | 3.1 |
| FI (%) | 6.9 | 4.06 | 43.9 |
| CE (%) | 98.27 | 100 | 94.5 |
| Parameters | Sample | ||
|---|---|---|---|
| Feed | Retentate at DV 3 | Retentate at VRF 2 | |
| TDS (° Brix) | 3.1 ± 0.4 | 0.7 ± 0.2 | 0.9 ± 0.5 |
| TC (g glucose/L) | 17.1 ± 0.4 | 6.2 ± 0.2 | 8.4 ± 0.5 |
| TPC (mg GAE/L) | 450 ± 1.3 | 432.4 ± 6.2 | 812.4 ± 10.2 |
| TAA (mM Trolox) | 3.0 ± 0.5 | 2.9 ± 0.6 | 4.8 ± 0.9 |
| Membrane Type | HFS | DCQ II-006 C-PS100 | GE | GH | GK |
|---|---|---|---|---|---|
| Manufacturer | Toray, Tokyo, Japan | China Blue Star Membrane Technology Co. Ltd., Beijing, China | GE Osmonics, Minnetonka, MN, USA | GE Osmonics, Minnetonka, MN, USA | GE Osmonics, Minnetonka, MN, USA |
| Membrane material | PVDF | PS | PA-TFC | PA-TFC | PA-TFC |
| Configuration | Hollow fiber | Hollow fiber | Flat-sheet | Flat-sheet/Spiral-wound | Flat-sheet |
| Nominal MWCO(Da) | - | 100,000 | 1000 | 2500 | 3500 |
| Pore size (m) | 0.02 | - | - | - | - |
| pH operating range | 2–12 | 2–13 | 2–10 | 2–10 | 2–10 |
| Max. operating temperature (°C) | 40 | 50 | 50 | 50 | 50 |
| Max. operating pressure (bar) | 2 | 1.5 | 27.6 | 27.6 | 27.6 |
| Membrane surface area (m2) | 0.4 | 0.16 | 0.0035 | 0.0035/0.32 | 0.0035 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conidi, C.; Drioli, E.; Cassano, A. Coupling Ultrafiltration-Based Processes to Concentrate Phenolic Compounds from Aqueous Goji Berry Extracts. Molecules 2020, 25, 3761. https://doi.org/10.3390/molecules25163761
Conidi C, Drioli E, Cassano A. Coupling Ultrafiltration-Based Processes to Concentrate Phenolic Compounds from Aqueous Goji Berry Extracts. Molecules. 2020; 25(16):3761. https://doi.org/10.3390/molecules25163761
Chicago/Turabian StyleConidi, Carmela, Enrico Drioli, and Alfredo Cassano. 2020. "Coupling Ultrafiltration-Based Processes to Concentrate Phenolic Compounds from Aqueous Goji Berry Extracts" Molecules 25, no. 16: 3761. https://doi.org/10.3390/molecules25163761