Cellulose Acetate Fabrics Loaded with Rhodamine B Hydrazide for Optical Detection of Cu(II)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fabrication of Cellulose Acetate Matrices
2.2. Loading of RBH into the Cellulose Acetate Fabrics: Morphological and Spectroscopic Characterization
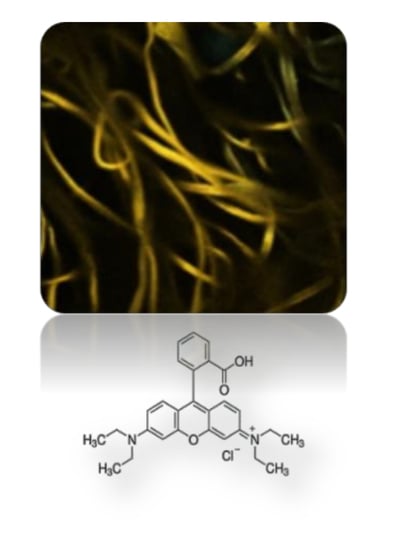
2.3. Fluorescence Confocal Laser Scanning Microscopy
3. Materials and Methods
3.1. Materials
3.2. Film Preparation by Solution Casting
3.3. Membrane Preparation by Phase Inversion
3.4. Fabrication of Nanofibers by Electrospinning
3.5. RBH Pre-Loaded Fabrics
3.6. RBH Post-Loaded Fabrics
3.7. Morphological Characterization
3.8. Spectroscopic and Photophysical Characterization
3.9. Fluorescence Confocal Laser Scanning Microscopy
3.10. Overview of the Studied Samples and Processes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Notes
References
- National Research Council (US) Committee on Copper in Drinking Water. Copper in Drinking Water. Washington (DC): National Academies Press (US); 2000. 5, Health Effects of Excess Copper. Available online: https://www.ncbi.nlm.nih.gov/books/NBK225400/ (accessed on 30 June 2020).
- Chen, X.; Pradhan, T.; Wang, F.; Kim, J.S.; Yoon, J. Fluorescent Chemosensors Based on Spiroring-Opening of Xanthenes and Related Derivatives. Chem. Rev. 2011, 112, 1910–1956. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Singh, A.; Tripathi, K.; Sonkar, A.K.; Chauhan, B.S.; Srikrishna, S.; James, T.D.; Mishra, L. A quick and selective rhodamine based “smart probe” for “signal-on” optical detection of Cu2+ and Al3+ in water, cell imaging, computational studies and solid state analysis. Sensors Actuat. B Chem. 2018, 266, 95–105. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, J.; Wang, R.; Chen, L. Highly sensitive and selective colorimetric and off-on fluorescent probe for Cu2+ based on rhodamine derivative. Org. Biomol. Chem. 2010, 8, 5277–5279. [Google Scholar] [CrossRef] [PubMed]
- López, M.T.; Pablos, J.L.; Muñoz, M.A.; Ibeas, S.; Serna, F.; García, F.C.; García, J.M. Aromatic polyamides and acrylic polymers as solid sensory materials and smart coated fibres for high acidity colorimetric sensing. Polym. Chem. 2015, 6, 3110–3120. [Google Scholar] [CrossRef] [Green Version]
- Favaretto, L.; Manoli, F.; Zambianchi, M.; Bocchi, L.; Ventura, B.; Manet, I.; Melucci, M. Immobilization of Perylene-3,4,9,10-Tetracarboxylic Dianhydride on Hollow Polysulfone Fibers: Primary Amine Coupling and Fluorescence Reporting. ChemPlusChem 2019, 84, 1299–1304. [Google Scholar] [CrossRef]
- Tao, C.; Ying, Y.-M.; Wang, H.; Chen, B.; Zhu, G.-P.; Song, Y.-J.; Liu, X.-G.; Zhao, Z.; Shen, L.; Tang, B.Z. Nonwoven fabric coated with a tetraphenylethene-based luminescent metal–organic framework for selective and sensitive sensing of nitrobenzene and ammonia. J. Mater. Chem. C 2018, 6, 12371–12376. [Google Scholar] [CrossRef]
- Liang, C.Z.; Chung, T.-S.; Lai, J.-Y. A review of polymeric composite membranes for gas separation and energy production. Prog. Polym. Sci. 2019, 97, 101141. [Google Scholar] [CrossRef]
- Hołda, A.K.; Vankelecom, I.F. Understanding and guiding the phase inversion process for synthesis of solvent resistant nanofiltration membranes. J. Appl. Polym. Sci. 2015, 132, 42130. [Google Scholar] [CrossRef]
- Rodríguez-Tobías, H.; Morales, G.; Grande, D. Comprehensive review on electrospinning techniques as versatile approaches toward antimicrobial biopolymeric composite fibers. Mater. Sci. Eng. C 2019, 101, 306–322. [Google Scholar] [CrossRef]
- Abrigo, M.; McArthur, S.L.; Kingshott, P. Electrospun Nanofibers as Dressings for Chronic Wound Care: Advances, Challenges, and Future Prospects. Macromol. Biosci. 2014, 14, 772–792. [Google Scholar] [CrossRef]
- Bedford, N.M.; Steckl, A.J. Photocatalytic Self Cleaning Textile Fibers by Coaxial Electrospinning. ACS Appl. Mater. Interfaces 2010, 2, 2448–2455. [Google Scholar] [CrossRef]
- Abdal-Hay, A.; Makhlouf, A.S.H.; Khalil, K.A.; Hamdy, A.S. Novel, Facile, Single-Step Technique of Polymer/TiO2Nanofiber Composites Membrane for Photodegradation of Methylene Blue. ACS Appl. Mater. Interfaces 2015, 7, 13329–13341. [Google Scholar] [CrossRef] [PubMed]
- Morsi, R.E.; ElSawy, M.A.; Ali, M.M.; Gentili, D.; Cavallini, M.; Manet, I.; Bandini, E.; Venturini, A.; Benaglia, M.; Ventura, B. Rhodamine B hydrazide loaded polysulfone fabrics for Cu(II) detection: Morphological and optical properties. J. Appl. Polym. Sci. 2019, 137, 48408. [Google Scholar] [CrossRef]
- Tungprapa, S.; Puangparn, T.; Weerasombut, M.; Jangchud, I.; Fakum, P.; Semongkhol, S.; Meechaisue, C.; Supaphol, P. Electrospun cellulose acetate fibers: Effect of solvent system on morphology and fiber diameter. Cellulose 2007, 14, 563–575. [Google Scholar] [CrossRef]
- Han, S.O.; Youk, J.H.; Min, K.D.; Kang, Y.O.; Park, W.H. Electrospinning of cellulose acetate nanofibers using a mixed solvent of acetic acid/water: Effects of solvent composition on the fiber diameter. Mater. Lett. 2008, 62, 759–762. [Google Scholar] [CrossRef]
- Cai, J.; Niu, H.; Yu, Y.; Xiong, H.; Lin, T. Effect of solvent treatment on morphology, crystallinity and tensile properties of cellulose acetate nanofiber mats. J. Text. Inst. 2016, 108, 1–8. [Google Scholar] [CrossRef]
- Kristoffersen, A.S.; Erga, S.R.; Hamre, B.; Frette, Ø. Testing Fluorescence Lifetime Standards using Two-Photon Excitation and Time-Domain Instrumentation: Rhodamine B, Coumarin 6 and Lucifer Yellow. J. Fluoresc. 2014, 24, 1015–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadkowski, P.; Fleming, G. Photophysics of the acid and base forms of rhodamine B. Chem. Phys. Lett. 1978, 57, 526–529. [Google Scholar] [CrossRef]
- Arbeloa, T.L.; Estévez, M.T.; Arbeloa, F.L.; Aguirresacona, I.U.; López-Arbeloa, I. Luminescence properties of rhodamines in water/ethanol mixtures. J. Lumin. 1991, 48, 400–404. [Google Scholar] [CrossRef]
- Tungsombatvisit, N.; Inprasit, T.; Rohmawati, D.; Pisitsak, P. Rhodamine Derivative- Based Cellulose Acetate Electrospun Colorimetric Sensor for Cu2+ Sensing in Water: Effects of Alkaline Treatment. Fibers Polym. 2019, 20, 481–489. [Google Scholar] [CrossRef]
- Silva, A.; Boto, R.E.; El-Shishtawy, R.M.; Almeida, P. Rhodamine B as ligand for affinity chromatography. Fixation studies onto cellulose by a curing method. Eur. Polym. J. 2006, 42, 2270–2282. [Google Scholar] [CrossRef]
- Che, M.; Védrine, J.C. Characterization of Solid Materials and Heterogeneous Catalysts: From Structure to Surface Reactivity; Wiley-VCH Verlag GmbH & Co. KGaA: New York, NY, USA, 2012; Volume 1. [Google Scholar]
- Lewkowicz, A.; Synak, A.; Grobelna, B.; Kułak, L.; Bojarski, P. Spectroscopic properties of Rhodamine B entrapped in hybrid porous nanolayers at high dye concentration. Chem. Phys. 2014, 439, 121–127. [Google Scholar] [CrossRef]
- Pasini, D.; Takeuchi, D. Cyclopolymerizations: Synthetic Tools for the Precision Synthesis of Macromolecular Architectures. Chem. Rev. 2018, 118, 8983–9057. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Zhang, Y.-K. Long-lived and largely red-shifted photoluminescence of solid-state rhodamine dyes: Molecular exciton coupling and structural effect. J. Lumin. 2015, 166, 215–221. [Google Scholar] [CrossRef]
- Kawahigashi, M.; Hirayama, S. Microscopic fluorescence decay measurements on thin liquid films and droplets of concentrated dye solutions. J. Lumin. 1989, 43, 207–212. [Google Scholar] [CrossRef]
- Sakai, Y.; Kawahigashi, M.; Minami, T.; Inoue, T.; Hirayama, S. Deconvolution of non-exponential emission decays arising from reabsorption of emitted light. J. Lumin. 1989, 42, 317–324. [Google Scholar] [CrossRef]
- Tedeschi, G.; Guzman-Puyol, S.; Paul, U.C.; Barthel, M.J.J.; Goldoni, L.; Caputo, G.; Ceseracciu, L.; Athanassiou, A.; Heredia-Guerrero, J.A. Thermoplastic cellulose acetate oleate films with high barrier properties and ductile behaviour. Chem. Eng. J. 2018, 348, 840–849. [Google Scholar] [CrossRef]
- Ghaseminezhad, S.M.; Barikani, M.; Salehirad, M. Development of graphene oxide-cellulose acetate nanocomposite reverse osmosis membrane for seawater desalination. Compos. Part B Eng. 2019, 161, 320–327. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |











| Sample | Solvent(s) Used for Preparation | Preparation Procedure | Loading with RBH (w/w%) a | Immersion Time with Cu(II) Solution | pH of the Cu(II) Solution | Cu(II) Concentration |
|---|---|---|---|---|---|---|
| Casted film | Acetone | Casting | 0.50% | - | - | - |
| 1.00% | - | - | - | |||
| Membrane | Acetone/DMF | Phase inversion | 0.50% | - | - | - |
| 1.00% | 1 night | 7 | 1, 10, 100, and 200 ppm | |||
| Nanofibers | DMF | Electrospinning | 0.50% | - | - | - |
| 1.00% | 1 night | 7 | 1, 10, 100, and 200 ppm | |||
| Nanofibers | DCM/Acetone | Electrospinning | 0.50% | - | - | - |
| 1.00% | 1 night | 7 | 1, 10, 100, and 200 ppm |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morsi, R.E.; Elsawy, M.; Manet, I.; Ventura, B. Cellulose Acetate Fabrics Loaded with Rhodamine B Hydrazide for Optical Detection of Cu(II). Molecules 2020, 25, 3751. https://doi.org/10.3390/molecules25163751
Morsi RE, Elsawy M, Manet I, Ventura B. Cellulose Acetate Fabrics Loaded with Rhodamine B Hydrazide for Optical Detection of Cu(II). Molecules. 2020; 25(16):3751. https://doi.org/10.3390/molecules25163751
Chicago/Turabian StyleMorsi, Rania E., Moataz Elsawy, Ilse Manet, and Barbara Ventura. 2020. "Cellulose Acetate Fabrics Loaded with Rhodamine B Hydrazide for Optical Detection of Cu(II)" Molecules 25, no. 16: 3751. https://doi.org/10.3390/molecules25163751