Resveratrol and Quercetin Administration Improves Antioxidant DEFENSES and reduces Fatty Liver in Metabolic Syndrome Rats
Abstract
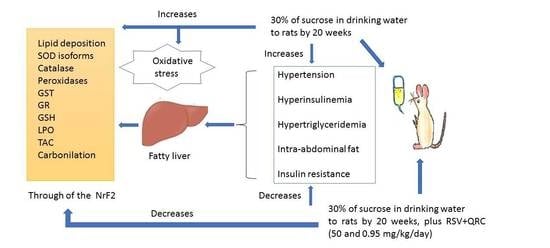
:1. Introduction
2. Materials and Methods
2.1. Animals and Diet
2.2. Serum Biochemistry Parameters
2.3. Lipid Detection in Fatty Liver
2.4. Liver Homogenization
2.5. Evaluation of Antioxidant Enzymatic System in Native Gels
2.6. Glutathione-S-Transferase and Glutathione Reductase Activities
2.7. Evaluation of Oxidative Stress Markers
2.7.1. Protein Carbonylation
2.7.2. Lipid Peroxidation
2.7.3. The Total Antioxidant Capacity (TAC)
2.7.4. Quantification of GSH Levels
2.8. Western Blotting of Nrf2
2.9. Statistical Analysis
3. Results
General Characteristics and Serum Biochemical Measurements.
Evaluation of Oxidative Stress Markers
Histopathological Findings
Antioxidant Enzymatic Activities
4. Discussion
4.1. Effect of RSV and QRC on Altered Biochemical Variables and Associated Signs in MS
4.2. Effect of RSV and QRC on NAFL and on Hepatic OS
4.3. Effect of RSV + QRC on Nrf2
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The interplay. BioMed. Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Ramírez, A.; Chávez-Salgado, M.; Peñeda-Flores, J.A.; Zapata, E.; Masso, F.; El-Hafidi, M. High-sucrose diet increases ROS generation, FFA accumulation, UCP2 level, and proton leak in liver mitochondria. Am. J. Physiol. Endocrinol. Metab. 2011. [CrossRef] [PubMed]
- Baños, G.; Medina-Campos, O.N.; Maldonado, P.D.; Zamora, J.; Pérez-Torres, I.; Pavón, N.; Pedraza-Chaverrí, J. Antioxidant enzymes in hypertensive and hypertriglyceridemic rats: Effect of gender. Clin. Exp. Hypertens. 2005, 27, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Dani, C.; Oliboni, L.S.; Pasquali, M.A.; Oliveira, M.R.; Umezu, F.M.; Salvador, M.; Moreira, J.C.; Henriques, J.A. Intake of purple grape juice as a hepatoprotective agent in Wistar rats. J. Med. Food. 2008, 11, 127–132. [Google Scholar] [CrossRef]
- Peredo-Escárcega, A.E.; Guarner-Lans, V.; Pérez-Torres, I.; Ortega-Ocampo, S.; Carreón-Torres, E.; Castrejón-Tellez, V.; Díaz-Díaz, E.; Rubio-Ruiz, M.E. The combination of resveratrol and quercetin attenuates metabolic syndrome in rats by modifying the serum fatty acid composition and by upregulating SIRT 1 and SIRT 2 expression in white adipose tissue. Evid. Based. Complement. Alternat. Med. 2015, 2015, 474032. [Google Scholar] [CrossRef]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, M.; Spangnuolo, C.; Tedesco, I.; Bilotto, S.; Russo, G. The flavonoid quercetin in disease prevention and therapy: Facts and fancies. Biochem. Pharmacol. 2012, 83, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Metabolic syndrome pandemic. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 629–636. [Google Scholar] [CrossRef]
- Patton, H.M.; Yates, K.; Unalp-Arida, A.; Behling, C.A.; Huang, T.T.; Rosenthal, P.; Sanyal, A.J.; Schwimmer, J.B.; Lavine, J.E. Association between metabolic syndrome and liver histology among children with nonalcoholic fatty liver disease. Am. J. Gastroenterol. 2010, 105, 2093–2102. [Google Scholar] [CrossRef]
- Wu, S.L.; Pan, C.; Yu, L.; Meng, K. Immunosuppression by Combined Use of cyclosporine and resveratrol in a rat liver transplantation model. Transplant. Proc. 2005, 37, 2354–2359. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hong, M.; Tan, H.Y.; Wang, N.; Feng, Y. Insights into the role and interdependence of oxidative stress and inflammation in liver diseases. Oxid. Med. Cell. Longev. 2016, 2016, 4234061. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Pérez-Torres, I.; Torres-Narváez, J.C.; Pedraza-Chaverri, J.; Rubio-Ruiz, M.E.; Díaz-Díaz, E.; Del Valle-Mondragón, L.; Martínez-Memije, R.; Varela López, E.; Guarner-Lans, V. Effect of the aged garlic extract on cardiovascular function in metabolic syndrome rats. Molecules 2016, 26, 1425. [Google Scholar]
- Fridovich, I. Oxygen toxicity: A radical explanation. J. Exp. Biol. 1998, 201, 1203–1209. [Google Scholar] [PubMed]
- Beutler, E. The relationship of red cell enzymes to red cell lifespan. Blood Cells 1988, 14, 69–75. [Google Scholar]
- Soto, M.E.; Zuñiga-Muñoz, A.; Guarner-Lans, V.; Duran-Hernández, E.J.; Pérez-Torres, I. Infusion of hibiscus sabdariffa L. Modulates oxidative stress in patients with marfan syndrome. Mediators. Inflamm. 2016, 2016, 8625203. [Google Scholar] [CrossRef]
- Zúñiga-Muñoz, A.M.; Pérez-Torres, I.; Guarner-Lans, V.; Núñez-Garrido, E.; Velázquez, E.R.; Huesca-Gómez, C.; Gamboa-Ávila, R.; Soto, M.E. Glutathione system participation in thoracic aneurysms from patients with Marfan syndrome. Vasa 2017, 46, 177–186. [Google Scholar] [CrossRef]
- Farghali, H.; Kgalalelo, K.M.; Wojnarová, L.; Kutinová, C.N. In vitro and in vivo experimental hepatotoxic models in liver research: Applications to the assessment of potential hepatoprotective drugs. Physiol. Res. 2016, 21, S417–S425. [Google Scholar]
- Cherniack, E.P. Polyphenols: Planting the seeds of treatment for the metabolic syndrome. Nutrition. 2011, 6, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Ruíz, M.E.; Del Valle-Mondragón, L.; Castrejón-Tellez, V.; Carreón-Torres, E.; Díaz-Díaz, E.; Guarner-Lans, V. Angiotensin II and 1-7 during aging in metabolic syndrome rats. Expression of AT1, AT2 and Mas receptors in abdominal white adipose tissue. Peptides 2014, 57, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Ghadge, A.A.; Khaire, A.A.; Kuvalekar, A.A. Adiponectin: A potential therapeutic target for metabolic syndrome. Cytokine Growth Factor Rev. 2018, 39, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Franco, J.G.; Dias-Rocha, C.P.; Fernandes, T.P.; Albuquerque, M.L.; Lisboa, P.C.; Moura, E.G.; Pazos-Moura, C.C.; Trevenzoli, I.H. Resveratrol treatment rescues hyperleptinemia and improves hypothalamic leptin signaling programmed by maternal high-fat diet in rats. Eur. J. Nutr. 2016, 55, 601–610. [Google Scholar] [CrossRef]
- Castrejón-Tellez, V.; Rodríguez-Pérez, J.M.; Pérez-Torres, I.; Pérez-Hernández, N.; Cruz-Lagunas, A.; Guarner-Lans, V.; Vargas-Alarcón, G.; Rubio-Ruiz, M.E. The Effect of resveratrol and quercetin treatment on PPAR mediated uncoupling protein (UCP-) 1, 2, and 3 expression in visceral white adipose tissue from metabolic syndrome rats. Int. J. Mol. Sci. 2016, 17, E1069. [Google Scholar] [CrossRef] [PubMed]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P. Perspective: Does brown fat protect against diseases of aging? Ageing Res. Rev. 2010, 9, 69–76. [Google Scholar] [CrossRef]
- Bagul, P.K.; Middela, H.; Matapally, S.; Padiya, R.; Bastia, T.; Madhusudana, K.; Reddy, B.R.; Chakravarty, S.; Banerjee, S.K. Attenuation of insulin resistance, metabolic syndrome and hepatic oxidative stress by resveratrol in fructose-fed rats. Pharmacol. Res. 2012, 66, 260–268. [Google Scholar] [CrossRef]
- Sheen, J.M.; Yu, H.R.; Tain, Y.L.; Tsai, W.L.; Tiao, M.M.; Lin, I.C.; Tsai, C.C.; Lin, Y.J.; Huang, L.T. Combined maternal and postnatal high-fat diet leads to metabolic syndrome and is effectively reversed by resveratrol: A multiple-organ study. Sci. Rep. 2018, 8, 5607. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Guertin, D.A.; Ali, S.M.; Sabatini, D.M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 2005, 307, 1098–1101. [Google Scholar] [CrossRef]
- Lipina, C.; Huang, X.; Finlay, D.; McManus, E.J.; Alessi, D.R.; Sutherland, C. Analysis of hepatic gene transcription in mice expressing insulin-insensitive GSK3. Biochem. J. 2005, 392, 633–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Li, J.; Zhang, Z.; Li, W.; Sun, Y.; Zhang, Q.; Feng, X.; Zhu, W. Effects of resveratrol on the amelioration of insulin resistance in KKAy mice. Can. J. Physiol. Pharmacol. 2012, 90, 237–242. [Google Scholar] [CrossRef]
- Takahashi, S.; Nakashima, Y. Repeated and long-term treatment with physiological concentrations of resveratrol promotes NO production in vascular endothelial cells. Br. J. Nutr. 2012, 107, 774–780. [Google Scholar] [CrossRef]
- Gordish, K.L.; Beierwaltes, W.H. Resveratrol induces acute endothelium-dependent renal vasodilation mediated through nitric oxide and reactive oxygen species scavenging. Am. J. Physiol. Renal. Physiol. 2014, 306, F542–F550. [Google Scholar] [CrossRef] [Green Version]
- Villegas-Romero, M.; Castrejón-Téllez, V.; Pérez-Torres, I.; Rubio-Ruiz, M.E.; Carreón-Torres, E.; Díaz-Díaz, E.; Del Valle-Mondragón, L.; Guarner-Lans, V. Short-Term Exposure to High Sucrose Levels near Weaning Has a Similar Long-Lasting Effect on Hypertension as a Long-Term Exposure in Rats. Nutrients 2018, 10, 728. [Google Scholar] [CrossRef]
- Li, H.; Förstermann, U. Pharmacological Prevention of eNOS Uncoupling. Curr. Pharm. Des. 2014, 20, 3595–3606. [Google Scholar] [CrossRef] [PubMed]
- Li, P.G.; Sun, L.; Han, X.; Ling, S.; Gan, W.T.; Xu, J.W. Quercetin induces rapid eNOS phosphorylation and vasodilation by an Akt-independent and PKA-dependent mechanism. Pharmacology 2012, 89, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Croft, K.D.; Hodgson, J.M.; Kyle, R.; Lee, I.L.; Wang, Y.; Stocker, R.; Ward, N.C. Quercetin and its metabolites improve vessel function by inducing eNOS activity via phosphorylation of AMPK. Biochem. Pharmacol. 2012, 84, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Dou, D.; Guo, Y.; Ying, L.; Liu, J.; Xu, X.; Yu, X.; Gao, Y. Inhibition of phosphoinositide 3-kinase potentiates relaxation of porcine coronary arteries induced by nitroglycerin by decreasing phosphodiesterase type 5 activity. Circ. J. 2012, 76, 230–237. [Google Scholar] [CrossRef]
- Benedict, M.; Zhang, X. Non-alcoholic fatty liver disease: An expanded review. World J. Hepatol. 2017, 9, 715–732. [Google Scholar] [CrossRef] [PubMed]
- Burgess, T.A.; Robich, M.P.; Sellke, F.W. Improving glucose metabolism with resveratrol in a swine model of metabolic syndrome through alteration of signaling pathways in the liver and skeletal muscle. Arch. Surg. 2011, 146, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Schmatz, R.; Perreira, L.B.; Stefanello, N.; Mazzanti, C.; Spanevello, R.; Gutierres, J.; Bagatini, M.; Martins, C.C.; Abdalla, F.H.; et al. Effects of resveratrol on biomarkers of oxidative stress and on the activity of delta aminolevulinic acid dehydratase in liver and kidney of streptozotocin-induced diabetic rats. Biochimie 2012, 94, 374–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.K.; Mukherjee, S.; Gupta, G.; Rao, D.N.; Vasudevan, D.M. Protective effect of resveratrol and vitamin E against ethanol-induced oxidative damage in mice: Biochemical and immunological basis. Indian. J. Biochem. Biophys. 2010, 47, 32–37. [Google Scholar]
- Kasdallah-Grissa, A.; Mornagui, B.; Aouani, E.; Hammami, M.; Gharbi, N.; Kamoun, A.; El-Fazaa, S. Protective effect of resveratrol on ethanol-induced lipid peroxidation in rats. Alcohol 2006, 41, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Palsamy, P.; Sivakumar, S.; Subramanian, S. Resveratrol attenuates hyperglycemia-mediated oxidative stress, proinflammatory cytokines and protects hepatocytes ultrastructure in streptozotocin-nicotinamide-induced experimental diabetic rats. Chem. Biol. Interact. 2010, 186, 200–210. [Google Scholar] [CrossRef]
- Arinç, E.; Yilmaz, D.; Bozcaarmutlu, A. Mechanism of inhibition of CYP1A1 and glutathione S-transferase activities in fish liver by quercetin, resveratrol, naringenin, hesperidin, and rutin. Nutr. Cancer 2015, 67, 137–144. [Google Scholar] [CrossRef]
- Guthrie, A.R.; Chow, H.S.; Martinez, J.A. Effects of resveratrol on drug- and carcinogen-metabolizing enzymes, implications for cancer prevention. Pharmacol. Res. Perspect. 2017, 5, e00294. [Google Scholar] [CrossRef]
- Hamadi, N.; Mansour, A.; Hassan, M.H.; Khalifi-Touhami, F.; Badary, O. Ameliorative effects of resveratrol on liver injury in streptozotocin-induced diabetic rats. J. Biochem. Mol. Toxicol. 2012, 26, 384–392. [Google Scholar] [CrossRef]
- Kasdallah-Grissa, A.; Mornagui, B.; Aouani, E.; Hammami, M.; El May, M.; Gharbi, N.; Kamoun, A.; El-Fazaâ, S. Resveratrol, a red wine polyphenol, attenuates ethanol-induced oxidative stress in rat liver. Life Sci. 2007, 80, 1033–1099. [Google Scholar] [CrossRef]
- Pérez-Torres, I.; Guarner-Lans, V.; and Rubio-Ruiz, M.E. Reductive stress in inflammation-associated diseases and the pro-Oxidant effect of antioxidant agents. Int. J. Mol. Sci. 2017, 18, 2098. [Google Scholar] [CrossRef]
- Kebieche, M.; Lakroun, Z.; Lahouel, M.; Bouayed, J.; Meraihi, Z.; Soulimani, R. Evaluation of epirubicin-induced acute oxidative stress toxicity in rat liver cells and mitochondria, and the prevention of toxicity through quercetin administration. Exp. Toxicol. Pathol. 2009, 61, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Jiang, X.; Hu, Y.; Zhang, Z. The protective role of resveratrol in the sodium arsenite-induced oxidative damage via modulation of intracellular GSH homeostasis. Biol. Trace. Elem. Res. 2013, 155, 119–131. [Google Scholar] [CrossRef]
- Myhrstad, M.C.; Carlsen, H.; Nordstrom, O.; Blomhoff, R.; Moskaug, J.O. Flavonoids increase the intracellular glutathione level by transactivation of the γ-glutamylcysteine synthetase catalytical subunit promoter. Free Radic. Biol. Med. 2002, 32, 386–393. [Google Scholar] [CrossRef]
- Arredondo, F.; Echeverry, C.; Abin-Carriquiry, J.A.; Blasina, F.; Antúnez, K.; Jones, D.P.; Go, Y.M.; Liang, Y.L.; Dajas, F. After cellular internalization, quercetin causes Nrf2 nuclear translocation, increases glutathione levels, and prevents neuronal death against an oxidative insult. Free Radic. Biol. Med. 2010, 49, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Granado-Serrano, A.B.; Martín, M.A.; Bravo, L.; Goya, L.; Ramos, S. Quercetin modulates Nrf2 and glutathione-related defenses in HepG2 cells: Involvement of p38. Chem. Biol. Interact. 2012, 195, 154–164. [Google Scholar] [CrossRef] [Green Version]
- Arús, B.A.; Souza, D.G.; Bellaver, B.; Souza, D.O.; Gonçalves, C.A.; Quincozes-Santos, A.; Bobermin, L.D. Resveratrol modulates GSH system in C6 astroglial cells through heme oxygenase 1 pathway. Mol. Cell. Biochem. 2017, 428, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Osorio, A.S.; González-Reyes, S.; Pedraza-Chaverri, J. Natural Nrf2 activators in diabetes. Clin. Chim. Acta 2015, 448, 182–192. [Google Scholar] [CrossRef]
- Javkhedkar, A.A.; Quiroz, Y.; Rodriguez-Iturbe, B.; Vaziri, N.D.; Lokhandwala, M.F.; Banday, A.A. Resveratrol restored Nrf2 function, reduced renal inflammation, and mitigated hypertension in spontaneously hypertensive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015. [CrossRef]
- Kulkarni, S.S.; Cantó, C. The molecular targets of resveratrol. Biochim. Biophys. Acta 2015, 1852, 1114–1123. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |







| MS RSV + QRC | MS | C | C RSV + QRC | |
|---|---|---|---|---|
| Weight (g) | 481.8 ± 7.7 | 598.1 ± 9.1 b | 457.2 ± 13.7 | 514.2 ± 20.9 |
| Intra-abdominal fat (g) | 8.9 ± 1.0 | 12.9 ± 0.3 b | 4.9 ± 0.3 | 6.3 ± 0.9 |
| Relative liver weight | 2.6±0.1 | 2.4±0.05 | 2.4±0.06 | 2.3±0.04 |
| SBP (mmHg) | 115.4 ± 2.9 | 141.8 ± 0.9 b | 102.8 ± 0.8 | 110.8 ± 3.9 |
| Glucose (mg/dL) | 90.1 ± 2.2 | 95.5 ± 2.4 | 93.0 ± 3.3 | 91.1 ± 5.2 |
| Cholesterol (mg/dL) | 61.3 ± 1.5 | 63.5 ± 3.2 | 53.7 ± 2.9 | 60.3 ± 2.8 |
| Triglycerides (mg/dL) | 96.1 ± 4.6 | 141.5 ± 4.2 b | 72.5 ± 5.1 | 85.2 ± 8.3 |
| Insulin (µU/mL) | 0.16 ± 0.01 | 0.45 ± 0.05 b | 0.16 ± 0.03 | 0.11 ± 0.02 |
| HOMA index | 0.60 ± 0.07 | 2.16 ± 0.30 b | 0.81 ± 0.19 | 0.46 ± 0.10 a |
| Leptin (ng/dL) | 3.9 ± 0.1 a | 4.8 ± 0.3 b | 2.3 ± 0.2 | 2.5 ± 0.1 |
| Adiponectin (μg/mL) | 5.5 ± 0.1 | 7.5 ± 0.4 b | 3.7 ± 0.1 | 3.4 ± 0.2 |
| mg of Protein | MS RSV + QRC | MS | C | C RSV + QRC |
|---|---|---|---|---|
| Carbonylation (ng of carbonyls) | 1.2 ± 0.2 | 2.2 ± 0.2 c | 1.8 ± 0.1 | 1.6 ± 0.3 |
| LPO (nmol of MDA) | 0.3 ± 0.1 | 0.9 ± 0.1c | 0.4 ± 0.1 b | 0.5 ± 0.1 |
| TAC (nmol of trolox) | 675.6 ± 23.6 | 592.0 ± 29.5 a | 741.7 ± 19.9 d | 689.0 ± 17.3 |
| GSH (nM) | 4.1 ± 0.026 | 3.1 ± 0.043 a | 4.9 ± 0.066 | 4.2 ± 0.029 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubio-Ruiz, M.E.; Guarner-Lans, V.; Cano-Martínez, A.; Díaz-Díaz, E.; Manzano-Pech, L.; Gamas-Magaña, A.; Castrejón-Tellez, V.; Tapia-Cortina, C.; Pérez-Torres, I. Resveratrol and Quercetin Administration Improves Antioxidant DEFENSES and reduces Fatty Liver in Metabolic Syndrome Rats. Molecules 2019, 24, 1297. https://doi.org/10.3390/molecules24071297
Rubio-Ruiz ME, Guarner-Lans V, Cano-Martínez A, Díaz-Díaz E, Manzano-Pech L, Gamas-Magaña A, Castrejón-Tellez V, Tapia-Cortina C, Pérez-Torres I. Resveratrol and Quercetin Administration Improves Antioxidant DEFENSES and reduces Fatty Liver in Metabolic Syndrome Rats. Molecules. 2019; 24(7):1297. https://doi.org/10.3390/molecules24071297
Chicago/Turabian StyleRubio-Ruiz, Maria Esther, Verónica Guarner-Lans, Agustina Cano-Martínez, Eulises Díaz-Díaz, Linaloe Manzano-Pech, Anel Gamas-Magaña, Vicente Castrejón-Tellez, Concepción Tapia-Cortina, and Israel Pérez-Torres. 2019. "Resveratrol and Quercetin Administration Improves Antioxidant DEFENSES and reduces Fatty Liver in Metabolic Syndrome Rats" Molecules 24, no. 7: 1297. https://doi.org/10.3390/molecules24071297