4. Experimental
4.1. General Experimental Details—Chemistry
Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker Avance 500 MHz instrument, and chemical shifts are given in ppm relative to Me
4Si with coupling constants (
J values) in Hz. Mass spectrometry was run in electrospray positive ionization mode (Bruker MicroTof instrument, Coventry, UK). Elemental analysis (% CHN) was run by combustion analysis through an outsourced service (Medac Ltd, Surrey, UK). Commercial compounds were used as received; 2-(4-bromophenyl)quinoline-4-carboxylic acid (
3a) [
20] and 2-(4-bromophenyl)-6-methoxyquinoline-4-carboxylic acid (
3b) [
21] were accessed via the standard Pfitzinger reaction protocol [
8].
4.2. Preparation of 2-(4-Bromophenyl)Quinoline-4-Carboxylic Acid Hydrazides (4a–b)
A mixture of the appropriate 2-(4-bromophenyl)quinoline-4-carboxylic acid (3a–b) (10 mmol), absolute ethanol (20 mL) and concentrated sulfuric acid (2 mL) was heated under reflux for 12 h. Excess ethanol was removed under reduced pressure and the resulting oil was rendered alkaline using aqueous sodium bicarbonate. The aqueous layer was extracted with dichloromethane (2 × 50 mL) and the combined organic extracts were dried over anhydrous sodium sulfate and concentrated under reduced pressure. The intermediate crude ester product was re-dissolved in ethanol (10 mL) and 98% hydrazine hydrate (10 mL) added. The solution was heated under reflux for a further 12 h and then allowed to cool to room temperature. The precipitate that formed was collected by filtration, washed with water (3 × 10 mL) to remove excess hydrazine hydrate, and dried in vacuo to give the intermediate quinoline carboxylic hydrazides (4a–b) in 75%–76% overall yield.
2-(4-Bromophenyl)quinoline-4-carboxylic acid hydrazide (4a). Yield 75%, m.p. 246–248 °C. 1H-NMR (DMSO-d6 ): 4.7 (s, 2H, NH2), 7.65 (t, 1H, ArCH), 7.79 (d, 2H, J = 8.9, ArCH), 7.87 (t, 1H, J = 7.5, ArCH), 8.13 (d, 2H, J = 2.9, ArCH), 8.24 (d, 1H, J = 5.7, CH aromatic), 8.27 (d, 2H, J = 8.9, ArCH), 10.02 (s, 1H, NH).
2-(4-Bromophenyl)-6-methoxyquinoline-4-carboxylic acid hydrazide (4b). Yield 76%, m.p. 266–268 °C. 1H-NMR (DMSO-d6 ): 3.9 (s, 3H, OCH3), 4.7 (s, 2H, NH2), 7.5 (d, 1H, J = 7.5, ArCH), 7.68 (d, 1H, J = 2.4, ArCH), 7.70 (d, 2H, J = 7.5, ArCH), 8.07 (d, 1H, J = 7.5, ArCH), 8.13 (s, 1H, ArCH), 8.27 (d, 2H, J = 7.5, ArCH), 10.09 (s, 1H, NH).
4.3. Preparation of 2-(2-(4-Bromophenyl)Quinoline-4-Carbonyl)-N-Arylhydrazine-1-Carbothioamides (5a–k)
To a solution of quinoline-4-carboxylic acid hydrazide (4a–b, 1 mmol) in absolute ethanol (20 mL) was added a solution of substituted phenylisothiocyanate (1 mmol) in ethanol (10 mL) with continuous stirring. The reaction mixture was heated under reflux for 12 h. After cooling to room temperature, the precipitate formed was collected by filtration, and washed with ice-cold ethanol (5 mL) to give the corresponding quinoline-4-carbonyl-N-arylhydrazine-1-carbothioamide (5a–k) which was used in the next step without further purification.
2-(2-(4-Bromophenyl)quinoline-4-carbonyl)-N-(4-chlorophenyl)hydrazine-1-carbothioamide (5a). Yield 74%, m.p. 247–249 °C. 1H-NMR (DMSO-d6): 7.45–7.62 (m, 4H, ArCH), 7.67 (t, 1H, J = 7.5, ArCH), 7.80 (d, 1H, J = 9.2, ArCH), 7.90 (d, 1H, J = 8.6, ArCH), 8.14 (d, 1H, J = 8.5, ArCH), 8.25 (d, 1H, J = 9.2, ArCH), 8.34 (d, 2H, J = 7.5, ArCH), 8.42 (d, 2H, J= 2.5, ArCH), 10.02 (s, 2H, NH), 10.95 (s, 1H, NH).
2-(2-(4-Bromophenyl)quinoline-4-carbonyl)-N-(3,4-dichlorophenyl)hydrazine-1-carbothioamide (5b). Yield 65%, m.p. 265–267 °C. 1H-NMR (DMSO-d6): 7.60 (m, 3H, ArCH), 7.77 (d, 1H, J = 8.5, ArCH), 7.85 (d, 2H, J = 8.5, ArCH), 8.09 (d, 1H, J = 7.5, ArCH), 8.19 (m, 1H, ArCH), 8.29 (m, 2H, ArCH), 8.42 (d, 2H, J = 7.5, ArCH), 10.18 (s, 1H, NH), 10.82 (s, 1H, NH), 11.28 (s, 1H, NH).
2-(2-(4-Bromophenyl)quinoline-4-carbonyl)-N-(4-methylphenyl)hydrazine-1-carbothioamide (5c). Yield 69%, m.p. 245–247 °C. 1H-NMR (DMSO-d6): 2.31 (s, 3H, CH3), 7.18 (d, 2H, J = 8.5, ArCH), 7.35 (s, 2H, ArCH), 7.70 (m, 1H, ArCH), 7.85 (m, 3H, ArCH), 8.16 (d, 1H, J = 8.5, ArCH), 8.26 (d, 2H, J = 8.47, ArCH), 8.41 (d, 1H, J = 8.5, ArCH), 8.46 (s, 1H, ArCH), 9.83 (s, 2H, NH), 10.86 (s, 1H, NH).
2-(2-(4-Bromophenyl)quinoline-4-carbonyl)-N-(4-nitrophenyl)hydrazine-1-carbothioamide (5d). Yield 75%, m.p. 255–257 °C. 1H-NMR (DMSO-d6): 7.72 (m, 1H, ArCH), 7.75 (d, 3H, J = 8.5, ArCH), 7.86 (m, 2H, ArCH), 8.15 (d, 1H, J = 8.5, ArCH), 8.33 (m, 4H, ArCH), 8.49 (d, 2H, J = 3.5, ArCH), 10.35 (s, 2H, NH), 11.09 (s, 1H, NH).
2-(2-(4-Bromophenyl)quinoline-4-carbonyl)-N-(4-methoxyphenyl)hydrazine-1-carbothioamide (5e). Yield 61%, m.p. 216–218 °C. 1H-NMR (DMSO-d6): 3.79 (s, 3H, OCH3), 6.85 (d, 2H, J = 7.8, ArCH), 7.35 (d, 2H, J = 2.5, ArCH), 7.70 (m, 1H, ArCH), 7.82 (d, 2H, J = 7.8, ArCH), 7.90 (d, 1H, J = 6.5, ArCH), 8.17 (d, 1H, J = 8.5, ArCH), 8.26 (d, 2H, J = 7.8, ArCH), 8.40 (d, 2H, J = 2.5, ArCH), 9.80 (s, 2H, NH), 10.45 (s, 1H, NH).
2-(2-(4-Bromophenyl)-6-methoxyquinoline-4-carbonyl)-N-(4-chlorophenyl)hydrazine-1-carbothioamide (5f). Yield 75%, m.p. 186–188 °C. 1H-NMR (DMSO-d6): 3.9 (s, 3H, OCH3), 7.48 (m, 5H, ArCH), 7.70 (d, 3H, J = 8.9, ArCH), 8.04 (d, 1H, J = 8.9, ArCH), 8.21 (d, 3H, J = 8.8, ArCH), 10.2 (s, 2H, NH), 10.89 (s, 1H, NH).
2-(2-(4-Bromophenyl)-6-methoxyquinoline-4-carbonyl)-N-(3,4-dichlorophenyl)hydrazine-1-carbothioamide (5g). Yield 73%, m.p. 199–201 °C. 1H-NMR (DMSO-d6): 3.9 (s, 3H, OCH3), 7.51 (m, 4H, ArCH), 7.56 (m, 2H, ArCH), 7.75 (d, 2H, J = 8.5, ArCH), 8.09 (d, 1H, J = 7.5, ArCH), 8.19 (d, 2H, J = 8.5, ArCH), 10.13 (s, 2H, NH), 11.28 (s, 1H, NH).
2-(2-(4-Bromophenyl)-6-methoxyquinoline-4-carbonyl)-N-(4-methylphenyl)hydrazine-1-carbothioamide (5h). Yield 65%, m.p. 209–211 °C. 1H-NMR (DMSO-d6): 2.30 (s, 3H, CH3), 3.90 (s, 3H, OCH3), 7.19 (d, 2H, J = 8.8, ArCH), 7.35 (s, 2H, ArCH), 7.53 (d, 2H, J = 7.2, ArCH), 7.69 (d, 3H, J = 7.5, ArCH), 8.03 (d, 1H, J = 7.5, ArCH), 8.23 (d, 2H, J = 7.5, ArCH), 9.83 (s, 2H, NH), 10.85 (s, 1H, NH).
2-(2-(4-Bromophenyl)-6-methoxyquinoline-4-carbonyl)-N-(4-nitrophenyl)hydrazine-1-carbothioamide (5i). Yield 71%, m.p. 175–177 °C. 1H-NMR (DMSO-d6): 3.90 (s, 3H, OCH3), 7.51 (d, 1H, J = 7.8, ArCH), 7.82 (d, 3H, J = 6.3, ArCH), 7.95 (s, 2H, ArCH), 8.10 (d, 1H, J = 9.0, ArCH), 8.30 (m, 5H, ArCH), 10.35 (s, 2H, ArCH), 11.09 (s, 1H, NH).
2-(2-(4-Bromophenyl)-6-methoxyquinoline-4-carbonyl)-N-(4-methoxyphenyl)hydrazine-1-carbothioamide (5j). Yield 70%, m.p. 207–209 °C. 1H-NMR (DMSO-d6): 3.75 (s, 3H, OCH3), 3.90 (s, 3H, OCH3), 6.90 (d, 2H, J = 7.5, ArCH), 7.37 (m, 2H, ArCH), 7.55 (d, 1H, J = 6.9, ArCH), 7.80 (d, 3H, J = 7.5, ArCH), 8.10 (d, 1H, J = 7.5, ArCH), 8.25 (d, 2H, J = 7.5, ArCH), 8.45 (s, 1H, ArCH), 9.80 (s, 2H, NH), 10.09 (s, 1H, NH). m/z (ES+) 537.04 (M+).
2-(2-(4-Bromophenyl)-6-methoxyquinoline-4-carbonyl)-N-(4-fluorophenyl)hydrazine-1-carbothioamide (5k). Yield 60%, m.p. 183–185 °C. 1H-NMR (DMSO-d6): 3.90 (s, 3H, OCH3), 7.19 (m, 2H, ArCH), 7.45 (m, 3H, ArCH), 7.76 (d, 3H, J = 7.9, ArCH), 8.12 (d, 1H, J = 8.6, ArCH), 8.23 (d, 2H, J = 7.6, ArCH), 8.43 (s, 1H, ArCH), 9.98 (s, 2H, NH), 10.89 (s, 1H, NH).
4.4. Preparation of {5-[2-(4-Bromophenyl)-Quinolin-4-yl}-N-Aryl-[1,3,4]Oxadiazol-2-Amines (6a-k)
A suspension of quinoline-4-carbonyl-N-arylhydrazine-1-carbothioamide (5a–k, 1 mmol) in 2N sodium hydroxide (10 mL) was heated under reflux for 3h. After cooling to room temperature, the crude product precipitate was collected by filtration. Recrystallisation from ethanol gave the corresponding pure quinolin-4-yl-N-aryl-[1,3,4]oxadiazol-2-amine (6a-k) in 40%–78% isolated yield.
{5-[2-(4-Bromophenyl)quinolin-4-yl}-N-(4-chlorophenyl)-[1,3,4]oxadiazol-2-amine (6a). Yield 76%, m.p. 254–257 °C. 1H-NMR (DMSO-d6): 7.49 (d, 2H, J = 6.9, ArCH), 7.72 (d, 2H, J = 7.2, ArCH), 7.83 (d, 3H, J = 7.2, ArCH), 7.95 (m, 1H, ArCH), 8.25 (m, 3H, ArCH), 8.5 (s, 1H, ArCH), 9.12 (d, 1H, J = 6.9, ArCH), 11.20 (s, 1H, NH). m/z (ES+) 478.10 (M+). Anal. (C23H14BrClN4O): % CHN required: C 57.82, H 2.95, N 11.73; found C 57.82, H 2.82, N 11.74.
{5-[2-(4-Bromophenyl)quinolin-4-yl}-N-(3,4-dichlorophenyl)-[1,3,4]oxadiazol-2-amine (6b). Yield 78%, m.p. 264–266 °C. 1H-NMR (DMSO-d6): 7.61 (dd, 1H, J = 7.5, 2.9, ArCH), 7.68 (d, 1H, J = 9.3, ArCH), 7.83 (m, 2H, ArCH), 7.88 (s, 1H, J = 2.5, ArCH), 7.94 (t, 1H, J = 7.5, ArCH), 8.04 (d, 1H, J = 2.7, ArCH), 8.22 (s, 1H, ArCH), 8.24 (d, 2H, J = 8.3, ArCH), 8.47 (s, 1H, ArCH), 9.13 (d, 1H, J = 7.8, ArCH), 11.38 (s, 1H, NH). m/z (ES+) 512.25 (M+). Anal. (C23H13BrCl2N4O): % CHN required: C 53.93, H 2.56, N 10.94; found C 53.59, H 2.68, N 10.67.
{5-[2-(4-Bromophenyl)quinolin-4-yl}-N-(4-methylphenyl)-[1,3,4]oxadiazol-2-amine (6c). Yield 40%, m.p. 265–267 °C. 1H-NMR (DMSO-d6): 2.96 (s, 3H, CH3), 7.23 (d, 2H, J = 8.2, ArCH), 7.6 (d, 2H, J = 8.2, ArCH), 7.70 (d, 2H, J = 8.5, ArCH), 7.95 (m, 1H, ArCH), 8.21 (d, 1H, J = 8.2, ArCH), 8.31 (d, 2H, J = 8.2, ArCH), 8.35 (d, 1H, J = 8.5, ArCH), 8.46 (s, 1H, ArCH), 9.15 (d, 1H, J = 8.5, ArCH), 10.86 (s, 1H, NH). 13C-NMR (DMSO-d6): 20.32 (CH3), 116.85, 117.36, 122.16, 123.86, 125.85, 128.37, 129.00, 129.10, 129.56, 129.99, 130.76, 131.24, 132.04, 135.81, 137.11, 148.32, 154.72, 156.20, 160.28. HRMS m/z (ES+) required 457.0586 (M++1), found 457.0589.
{5-[2-(4-Bromophenyl)quinolin-4-yl}-N-(4-nitrophenyl)-[1,3,4]oxadiazol-2-amine (6d). Yield 59%, m.p. 237–239 °C. 1H-NMR (DMSO-d6): 7.86 (d, 3H, J = 8.5, ArCH), 7.98 (m, 3H, ArCH), 8.27 (m, 3H, ArCH), 8.35 (d, 2H, J = 8.5, ArCH), 8.50 (s, 1H, ArCH), 9.15 (d, 1H, J = 7.5, ArCH), 11.84 (s, 1H, NH). m/z (ES+) 489.25 (M+). Anal. (C23H14BrN5O3): % CHN required: C 56.57, H 2.89, N 14.34; found C 56.59, H 2.79, N 14.37.
{5-[2-(4-Bromophenyl)quinolin-4-yl}-N-(4-methoxyphenyl)-[1,3,4]oxadiazol-2-amine (6e). Yield 65%, m.p. 229–231 °C. 1H-NMR (DMSO-d6): 3.79 (s, 3H, OCH3), 7.00 (d, 2H, J = 8.9, ArCH), 7.56 (d, 2H, J = 8.9, ArCH), 7.97 (m, 3H, ArCH), 7.92 (m, 1H, ArCH), 8.20 (m, 3H, ArCH), 8.50 (s, 1H, ArCH), 9.17 (d, 1H, J = 7.5, ArCH), 10.78 (s, 1H, NH). m/z (ES+) 474.05 (M+). Anal. (C24H17BrN4O2): % CHN required: C 60.90, H 3.62, N 11.84; found C 60.69, H 3.33, N 11.72.
{5-[2-(4-Bromophenyl)-6-methoxyquinolin-4-yl}-N-(4-chlorophenyl)-[1,3,4]oxadiazol-2-amine (6f). Yield 48%, m.p. 261–263 °C. 1H-NMR (DMSO-d6): 3.97 (s, 3H, OCH3), 7.41 (d, 2H, J = 6.9, ArCH), 7.54 (d, 1H, J = 7.6, ArCH), 7.63 (d, 2H, J = 6.9, ArCH), 7.75 (d, 2H, J = 7.45, ArCH), 8.15 (d, 1H, J = 8.5, ArCH), 8.24 (d, 2H, J = 8.5, ArCH), 8.40 (s, 1H, ArCH), 8.7 (d, 1H, J = 2.5, ArCH). m/z (ES+) 508.02 (M+). Anal. (C24H16BrClN4O2): % CHN required: C 56.77, H 3.18, N 11.03; found C 56.83, H 3.12, N 11.13.
{5-[2-(4-Bromophenyl)-6-methoxyquinolin-4-yl}-N-(3,4-dichlorophenyl)-[1,3,4]oxadiazol-2-amine (6g). Yield 59%, m.p. 227–229 °C. 1H-NMR (DMSO-d6): 3.95 (s, 3H, OCH3), 7.18 (d, 2H, J = 7.3, ArCH), 7.52 (d, 1H, J = 6.3, ArCH), 7.78 (d, 2H, J = 8.0, ArCH), 7.89 (d, 1H, J = 2.5, ArCH), 8.09 (d, 2H, J = 8.3, ArCH), 8.29 (d, 2H, J = 7.3, ArCH), 8.31 (s, 1H, NH), 8.90 (d, 1H, J = 2.3, ArCH). m/z (ES+) 541.98 (M+). Anal. (C24H15BrCl2N4O2): % CHN required: C 53.16, H 2.79, N 10.33; found C 52.92, H 2.86, N 10.12.
{5-[2-(4-Bromophenyl)-6-methoxyquinolin-4-yl}-N-(4-methylphenyl)-[1,3,4]oxadiazol-2-amine (6h). Yield 54%, m.p. 239–241 °C. 1H-NMR (DMSO-d6): 2.2 (s, 3H, CH3), 4.0 (s, 3H, OCH3), 7.20 (d, 2H, J = 8.3, ArCH), 7.62 (d, 3H, J = 8.7, ArCH), 7.75 (d, 2H, J = 9.1, ArCH), 8.09 (d, 1H, J = 9.1, ArCH), 8.2 (d, 2H, J = 8.3, ArCH), 8.40 (s, 1H, ArCH), 8.65 (d, 1H, J = 3.0, ArCH), 10.85 (s, 1H, NH). HRMS m/z (ES+) required 487.0691 (M++1), found 487.0693.
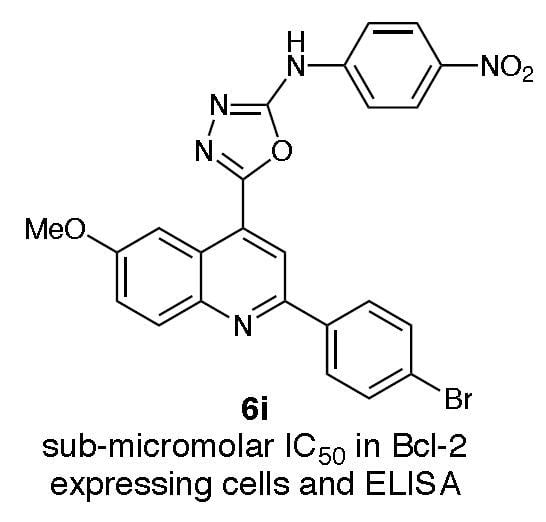
{5-[2-(4-Bromophenyl)-6-methoxyquinolin-4-yl}-N-(4-nitrophenyl)-[1,3,4]oxadiazol-2-amine (6i). Yield 45%, m.p. 272–274 °C. 1H-NMR (DMSO-d6): 3.99 (s, 3H, OCH3), 7.55 (dd, 1H, J = 9.0, ArCH), 7.64 (d, 2H, J = 9.0, ArCH), 7.78 (d, 2H, J = 9.0, ArCH), 7.96 (s, 1H, ArCH), 8.10 (d, 3H, J = 9.2, ArCH), 8.24 (d, 2H, J = 8.6, ArCH), 8.39 (s, 1H, NH), 8.79 (d, 1H, J = 3.1, ArCH). HRMS (C24H16BrN5O4) m/z (ES+) required 517.0380 (M++1), found 517.0428. Anal. (C24H16BrN5O4): % CHN required: C 56.10, H 3.27; found C 55.90, H 3.11.
{5-[2-(4-Bromophenyl)-6-methoxyquinolin-4-yl}-N-(4-methoxyphenyl)-[1,3,4]oxadiazol-2-amine (6j). Yield 57%, m.p. 232–234 °C. 1H-NMR (DMSO-d6): 3.69 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 6.90 (d, 2H, J = 7.3, ArCH), 7.58 (m, 3H, ArCH), 7.80 (d, 2H, J = 7.9, ArCH), 8.10 (d, 1H, J = 7.3, ArCH), 8.19 (d, 2H, J = 7.9, ArCH), 8.40 (s, 1H, ArCH), 8.54 (d, 1H, J = 2.5, ArCH). m/z (ES+) 505.07 (M+). Anal. (C25H19BrN4O3): % CHN required: C 59.65, H 3.80, N 11.13; found C 59.35, H 3.68, N 10.91.
{5-[2-(4-Bromophenyl)-6-methoxyquinolin-4-yl}-N-(4-fluorophenyl)-[1,3,4]oxadiazol-2-amine (6k). Yield 47%, m.p. 230–232 °C. 1H-NMR (DMSO-d6): 3.98 (s, 3H, OCH3), 7.12 (m, 2H, ArCH), 7.54 (d, 1H, J = 8.5, ArCH), 7.62 (m, 2H, ArCH), 7.78 (d, 2H, J = 8.5, ArCH), 8.08 (d, 1H, J = 8.5, ArCH), 8.19 (d, 2H, J = 7.8, ArCH), 8.31 (s, 1H, ArCH), 8.75 (d, 1H, J = 2.9, ArCH). m/z (ES+) 492.07 (M+). Anal. (C24H16BrFN4O2): % CHN required: C 59.01, H 3.68, N 10.90; found C 58.67, H 3.28, N 11.20.
4.5. Preparation of 5-[2-(4-Bromophenyl)-6-methoxyquinolin-4-yl]-4-aryl-4H-[1,2,4]triazole-3-thiols (7a–b).
A solution of 2-(2-(4-bromophenyl)-6-methoxyquinoline-4-carbonyl)-N-(4-aryl)hydrazine-1-carbothioamide (10 mmol) in 2N NaOH was heated under reflux for 3 h. After cooling to room temperature, water was added and the mixture carefully neutralized with 0.1M HCl. The precipitate formed was filtered, dried and recrystallized from ethanol to give the corresponding triazole thiol product (7a–b).
5-[2-(4-Bromophenyl)-6-methoxyquinolin-4-yl]-4-(4-methoxyphenyl)-4H-[1,2,4]triazole-3-thiol (
7a). Yield 58%, m.p. 199–201 °C.
1H-NMR (DMSO-
d6): 3.70 (s, 3H, OCH
3), 3.87 (s, 3H, OCH
3), 6.86 (d, 2H,
J = 8.9, ArCH), 7.14 (d, 2H,
J = 8.9, ArCH), 7.41 (dd, 1H,
J = 9.3, ArCH), 7.51 (s, 1H, ArCH), 7.65 (d, 2H,
J = 8.5, ArCH), 7.83 (m, 3H, ArCH), 7.95 (d, 1H,
J = 9.2, ArCH).
5-[2-(4-Bromophenyl)-6-methoxyquinolin-4-yl]-4-(3,4-dichlorophenyl)-4H-[1,2,4]triazole-3-thiol (
7b). Yield 64%, m.p. 170–172 °C.
1H-NMR (DMSO-
d6): 3.86 (s, 3H, OCH
3), 7.08 (dd, 1H,
J = 8.3, ArCH), 7.42 (dd, 2H,
J = 9.2, ArCH), 7.49 (d, 1H,
J = 8.3, ArCH), 7.61 (d, 1H,
J = 2.6, ArCH), 7.67 (d, 2H,
J = 8.8, ArCH), 7.82 (d, 1H,
J = 2.4, ArCH), 7.93 (d, 2H,
J = 8.8, ArCH), 7.97 (d, 1H,
J = 9.4, ArCH).
4.6. Preparation of 5-[2-(4-Bromophenyl)-6-methoxyquinolin-4-yl]-4-aryl-4H-[1,2,4]triazole-3-ylsulfanyl]-1-phenylethanone (8a–b).
2-Bromoacetophenone (5 mmol) was added to a solution of 5-[2-(4-bromophenyl)-6-methoxyquinolin-4-yl]-4-aryl-4
H-[
1,
2,
4]triazine-3-thiol (
7a–b, 5 mmol) in ethanol (70%) containing KOH (5 mmol). The reaction mixture was left to stir at room temperature for 16 h. Water was then added and the precipitate formed was filtered, dried and recrystallized from ethanol to give the corresponding S-alkyl triazole thiol product (
8a–b).
5-[2-(4-Bromophenyl)-6-methoxyquinolin-4-yl]-4-(4-methoxyphenyl)-4H-[1,2,4]triazole-3-ylsulfanyl]-1-phenylethanone (
8a). Yield 63%, m.p. 240–242 °C.
1H-NMR (DMSO-
d6): 3.70 (s, 3H, OCH
3), 3.87 (s, 3H, OCH
3), 5.42 (s, 2H, CH
2), 6.86 (d, 2H,
J = 8.9, ArCH), 7.14 (d, 1H,
J = 8.9, ArCH), 7.46 (m, 5H, ArCH), 7.51 (m, 2H, ArCH), 7.62 (d, 2H,
J = 9.5, ArCH), 7.83 (m, 2H, ArCH), 8.15 (m, 3H, ArCH).
m/
z (ES
+) 639.08 (M
+).
5-[2-(4-Bromophenyl)-6-methoxyquinolin-4-yl]-4-(3,4-dichlorophenyl)-4H-[1,2,4]triazole-3-ylsulfanyl]-1-phenylethanone (
8b). Yield 63%, m.p. 247–249 °C.
1H-NMR (DMSO-
d6): 3.70 (s, 3H, OCH
3), 5.12 (s, 2H, CH
2), 7.32 (d, 1H,
J = 2.3, ArCH), 7.54 (m, 2H, ArCH), 7.62 (m, 2H, ArCH), 7.76 (m, 4H, ArCH), 8.10 (m, 6H, ArCH), 8.16 (s, 1H, ArCH). Anal. (C
32H
21BrCl
2N
4O
2S): % CHN required: C 56.82, H 3.13, N 8.28; found C 57.31, H 3.03, N 8.43.
4.7. Cell Viability—MTT Assay (MDA-MB-231 and HeLa Cells)
Human breast cancer MDA-MB-231 cells were cultured in RPMI 1640 medium (Life Technologies, Paisley, UK) supplemented with 10% foetal bovine serum, 100 IU/mL pencillin, and 100 μg/mL streptomycin (Life Technologies). Human cervical cancer HeLa cells were cultured in D-MEM (Life Technologies) supplemented with 10% foetal bovine serum, 100 IU/mL pencillin, and 100 μg/mL streptomycin (Life Technologies). Cells were passaged routinely and maintained at 37 °C and 5% CO2. For each experiment, 3000 cells in 0.2 mL of medium were seeded into each well of a clear flat-bottomed 96-well plate and allowed to adhere for 24 h. The cells were then incubated with test compound (from 10 mM stock in DMSO) over a 10 fold dilution series with concentrations ranging from 0.00001 mM to 100 μM, each diluent being performed in triplicate. Control experiments were conducted using DMSO vehicle control with volumes equivalent to test compound concentrations. Cells were incubated with test compound for 72 h., followed by treatment of wells with 20 μL of 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) solution (from a freshly made 5.5 μg/mL stock in PBS, Sigma-Aldrich, Gillingham, Dorset, UK). After 4 hours the solutions from the wells were removed and the resulting formazan crystals were dissolved in DMSO (200 μL) and incubated for 30 minutes at 37 °C. Absorbance was measured at 550 nm using an automated microplate reader. Mean IC50 values were obtained from plots of absorbance versus test compound concentration using GraphPad Prism 5 software (San Diego, CA, USA). For each concentration, three independent repeat experiments were carried out to establish reproducibility.
4.8. Cell Viability—CellTiter-Blue® Assay (KG1a and Jurkat Cells)
Human acute myeloid leukaemia KG1a cells and acute T-cell lymphocytic Jurkat cells were cultured in RPMI 1640 medium (Life Technologies, Paisley, UK) supplemented with 10% foetal bovine serum, 100 IU/mL pencillin, and 100 μg/mL streptomycin (Life Technologies). Cells were passaged routinely and maintained at 37 °C and 5% CO2. For each experiment 15,000 cells in 0.1 mL of medium were seeded into each well of a solid black fluorescence 96-well plate and incubated for 24 h. The cells were then incubated with test compound (from 10 mM stock in DMSO) over a 10-fold dilution series with concentrations ranging from 0.00001 mM to 100 μM, each diluent being performed in triplicate. Control experiments were conducted using DMSO vehicle control with volumes equivalent to test compound concentrations. Cells were incubated with test compound for 24 h, followed by treatment with 20 μL of CellTiter-Blue® (Promega, Southampton, UK). Fluorescence was measured using 544 nm excitation and 590 nm emission filters on a fluorescent plate reader. Mean IC50 values were obtained from plots of absorbance versus test compound concentration using GraphPad Prism 5 software (San Diego, CA, USA). For each concentration, three independent repeat experiments were carried out to establish reproducibility.
4.9. Enzyme-Linked Immunosorbent Assay (ELISA)
A streptavidin-coated 96-well microtitre plate (Thermo Scientific, Rockford, IL) was washed three times with PBS containing 0.05% Tween-20. Biotinylated Bim peptide (residues 81–106) was diluted to 0.09 μg/mL (20 nM) in Superblock blocking buffer in PBS (Thermo Scientific), and aliquots (200 μL) were transferred to each well. Following incubation (1.5 h) at room temperature to allow immobilization of the Bim peptide on to the solid surface (via streptavidin-biotin interaction), the plate was washed three times with 0.5% BSA in PBS containing 0.05% Tween-20. Test compounds were dissolved in DMSO to obtain a 50 mM stock solution, and different concentrations of tested compound (10 fold dilution series from stock solution) were incubated with 20 nM His-tagged Bcl-2 protein (Abcam, Cambridge, UK) in PBS for one hour. The inhibitor and protein mixture (100 μL) were then transferred to the wells containing bound Bim peptide and incubated at room temperature for two hours. Following further washing of the plate in triplicate (0.5% BSA in PBS containing 0.05% Tween-20), anti-His antibody containing horseradish peroxidase enzyme (Qiagen, Crawley, UK) was diluted in 0.5% BSA in PBS (1:1000), and aliquots of 100 μL were added to each well. After incubation (one hour) at room temperature, the plate was washed three times as previously to remove any unbound antibody. A 200 μM solution of o-phenylenediamine (Sigma-Aldrich, UK) was freshly prepared at pH5 using phosphate-citrate buffer (Sigma-Aldrich), and hydrogen peroxide was added to the solution to give a final concentration of 0.004 %. Aliquots (200 μL) of the prepared solution were added to the wells and the plate incubated in the dark for 30 minutes at room temperature. The optical density was then read using a plate reader at 450 nm. The experiments were carried on three separate occasions, including both negative and positive controls, where the negative control contains all the components except the Bcl-2 protein, whereas the positive control contains all the components except the inhibitors.
The reduction of affinity of the Bim peptide for Bcl-2 was calculated as:
% Reduction in Bim affinity = l450 of treated wells (with inhibitor)/l450 of positive control × 100
A plot of log μM concentration for each inhibitor against the percentage reduction in affinity for the Bim peptide was created using non-linear regression curve analysis (GraphPad Prism 5), and the software used to generate the IC50 value for the Bcl-2 inhibition.
4.10. Molecular Modeling and Docking
All molecular modeling studies were performed on a RM Innovator Pentium IV (2.4 GHz) running Linux Fedora Core 3. The protein crystal structure of Bcl-2 was downloaded from (
http://www.rcsb.org/pdb code: 4AQ3). Hydrogen atoms were added to the protein, using the protonate 3D option in MOE (Molecular Operating Environment). Ligand structures were built within MOE and energy minimized using the MMFF94x force field until a RMSD gradient of 0.05 Kcal mol
-1 A°
-1 was reached. The defined pocket was taken as the active site; Alpha triangle was chosen as the replacement methodology, London dG as the scoring function and ten conformations were retained for each compound.