Biological Role of Gellan Gum in Improving Scaffold Drug Delivery, Cell Adhesion Properties for Tissue Engineering Applications
Abstract
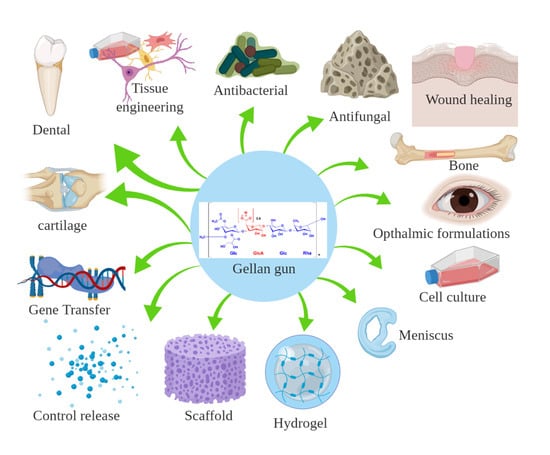
:1. Introduction
2. Gellan Gum in Drug Delivery
3. Gellan Gum Cell Adhesion Properties
4. Conclusion
Funding
Conflicts of Interest
References
- Maji, K.; Dasgupta, S.; Pramanik, K.; Bissoyi, A. Preparation and Evaluation of Gelatin-Chitosan-Nanobioglass 3D Porous Scaffold for Bone Tissue Engineering. Int. J. Biomater. 2016, 2016, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansson, P.-E.; Lindberg, B.; Sandford, P.A. Structural studies of gellan gum, an extracellular polysaccharide elaborated by Pseudomonas elodea. Carbohydr. Res. 1983, 124, 135–139. [Google Scholar] [CrossRef]
- Osmalek, T.; Froelich, A.; Tasarek, S. Application of gellan gum in pharmacy and medicine. Int. J. Pharm. 2014, 466, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Bacelar, A.H.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L. Recent progress in gellan gum hydrogels provided by functionalization strategies. J. Mater. Chem. B 2016, 4, 6164–6174. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, S.; Jana, S.; Gandhi, A.; Sen, K.K.; Zhiang, W.; Kokare, C. Gellan gum microspheres containing a novel alpha-amylase from marine Nocardiopsis sp. strain B2 for immobilization. Int. J. Biol. Macromol. 2014, 70, 292–299. [Google Scholar] [CrossRef]
- Mahdi, M.H.; Conway, B.R.; Smith, A.M. Development of mucoadhesive sprayable gellan gum fluid gels. Int. J. Pharm. 2015, 488, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Rosas-Flores, W.; Ramos-Ramirez, E.G.; Salazar-Montoya, J.A. Microencapsulation of Lactobacillus helveticus and Lactobacillus delbrueckii using alginate and gellan gum. Carbohydr. Polym. 2013, 98, 1011–1017. [Google Scholar] [CrossRef]
- Salunke, S.R.; Patil, S.B. Ion activated in situ gel of gellan gum containing salbutamol sulphate for nasal administration. Int. J. Biol. Macromol. 2016, 87, 41–47. [Google Scholar] [CrossRef]
- Warren, H.; in het Panhuis, M. Highly conducting composite hydrogels from gellan gum, PEDOT:PSS and carbon nanofibres. Synth. Met. 2015, 206, 61–65. [Google Scholar] [CrossRef]
- Wang, F.; Wen, Y.; Bai, T. The composite hydrogels of polyvinyl alcohol-gellan gum-Ca(2+) with improved network structure and mechanical property. Mat. Sci. Eng. C Mater. 2016, 69, 268–275. [Google Scholar] [CrossRef]
- Kang, D.; Zhang, H.-B.; Nitta, Y.; Fang, Y.-P.; Nishinari, K. Gellan. In Polysaccharides: Bioactivity and Biotechnology; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1627–1682. [Google Scholar]
- Karthika, J.S.; Vishalakshi, B. Novel stimuli responsive gellan gum-graft-poly(DMAEMA) hydrogel as adsorbent for anionic dye. Int. J. Biol. Macromol. 2015, 81, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Pacelli, S.; Paolicelli, P.; Dreesen, I.; Kobayashi, S.; Vitalone, A.; Casadei, M.A. Injectable and photocross-linkable gels based on gellan gum methacrylate: A new tool for biomedical application. Int. J. Biol. Macromol. 2015, 72, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Sonje, A.G.; Mahajan, H.S. Nasal inserts containing ondansetron hydrochloride based on Chitosan–gellan gum polyelectrolyte complex: In vitro–in vivo studies. Mat. Sci. Eng. C 2016, 64, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Prezotti, F.G.; Cury, B.S.; Evangelista, R.C. Mucoadhesive beads of gellan gum/pectin intended to controlled delivery of drugs. Carbohydr. Polym. 2014, 113, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Das, A.; Nayak, A.K.; Sen, K.K.; Basu, S.K. Aceclofenac-loaded unsaturated esterified alginate/gellan gum microspheres: In vitro and in vivo assessment. Int. J. Biol. Macromol. 2013, 57, 129–137. [Google Scholar] [CrossRef]
- Goyal, R.; Tripathi, S.K.; Tyagi, S.; Ravi Ram, K.; Ansari, K.M.; Shukla, Y.; Kar Chowdhuri, D.; Kumar, P.; Gupta, K.C. Gellan gum blended PEI nanocomposites as gene delivery agents: Evidences from in vitro and in vivo studies. Eur. J. Pharm. Biopharem. 2011, 79, 3–14. [Google Scholar] [CrossRef]
- Morris, E.R.; Nishinari, K.; Rinaudo, M. Gelation of gellan—A review. Food Hydrocoll. 2012, 28, 373–411. [Google Scholar] [CrossRef]
- Grasdalen, H.; Smidsrød, O. Gelation of gellan gum. Carbohydr. Polym. 1987, 7, 371–393. [Google Scholar] [CrossRef]
- Smith, A.M.; Shelton, R.M.; Perrie, Y.; Harris, J.J. An initial evaluation of gellan gum as a material for tissue engineering applications. J. Biomater. Appl. 2007, 22, 241–254. [Google Scholar] [CrossRef]
- Dentini, M.; Desideri, P.; Crescenzi, V.; Yuguchi, Y.; Urakawa, H.; Kajiwara, K. Synthesis and Physicochemical Characterization of Gellan Gels. Macromolecules 2001, 34, 1449–1453. [Google Scholar] [CrossRef]
- Yuguchi, Y.; Urakawa, H.; Kitamura, S.; Wataoka, I.; Kajiwara, K. The sol-gel transition of gellan gum aqueous solutions in the presence of various metal salts. In Physical Chemistry and Industrial Application of Gellan Gum; Nishinari, K., Ed.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 41–47. [Google Scholar]
- Oliveira, J.T.; Martins, L.; Picciochi, R.; Malafaya, P.B.; Sousa, R.A.; Neves, N.M.; Mano, J.F.; Reis, R.L. Gellan gum: A new biomaterial for cartilage tissue engineering applications. J. Biomed. Mater. Res. 2010, 93, 852–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, J.T.; Gardel, L.S.; Rada, T.; Martins, L.; Gomes, M.E.; Reis, R.L. Injectable gellan gum hydrogels with autologous cells for the treatment of rabbit articular cartilage defects. J. Ortho. Res. 2010, 28, 1193–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matricardi, P.; Cencetti, C.; Ria, R.; Alhaique, F.; Coviello, T. Preparation and characterization of novel gellan gum hydrogels suitable for modified drug release. Molecules 2009, 14, 3376–3391. [Google Scholar] [CrossRef] [PubMed]
- Babu, R.J.; Sathigari, S.; Kumar, M.T.; Pandit, J.K. Formulation of controlled release gellan gum macro beads of amoxicillin. Curr. Drug Deliv. 2010, 7, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, S.; Aoyama, H.; Kawasaki, N.; Kubo, W.; Attwood, D. In situ-gelling gellan formulations as vehicles for oral drug delivery. J. Controlled Release 1999, 60, 287–295. [Google Scholar] [CrossRef]
- Pereira, D.R.; Silva-Correia, J.; Caridade, S.G.; Oliveira, J.T.; Sousa, R.A.; Salgado, A.J.; Oliveira, J.M.; Mano, J.F.; Sousa, N.; Reis, R.L. Development of gellan gum-based microparticles/hydrogel matrices for application in the intervertebral disc regeneration. Tissue Eng. Part. C Methods 2011, 17, 961–972. [Google Scholar] [CrossRef] [Green Version]
- van Uden, S.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L. Current strategies for treatment of intervertebral disc degeneration: Substitution and regeneration possibilities. Biomater. Res. 2017, 21, 22. [Google Scholar] [CrossRef] [Green Version]
- Pereira, D.R.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L.; Pandit, A.; Biggs, M.J. Nanocellulose reinforced gellan-gum hydrogels as potential biological substitutes for annulus fibrosus tissue regeneration. Nanomedicine 2018, 14, 897–908. [Google Scholar] [CrossRef]
- Manda, M.G.; da Silva, L.P.; Cerqueira, M.T.; Pereira, D.R.; Oliveira, M.B.; Mano, J.F.; Marques, A.P.; Oliveira, J.M.; Correlo, V.M.; Reis, R.L. Gellan gum-hydroxyapatite composite spongy-like hydrogels for bone tissue engineering. J. Biomed. Mater. Res. 2018, 106, 479–490. [Google Scholar] [CrossRef] [Green Version]
- Gantar, A.; da Silva, L.P.; Oliveira, J.M.; Marques, A.P.; Correlo, V.M.; Novak, S.; Reis, R.L. Nanoparticulate bioactive-glass-reinforced gellan-gum hydrogels for bone-tissue engineering. Mat. Sci. Eng. C-Mater. 2014, 43, 27–36. [Google Scholar] [CrossRef]
- Douglas, T.E.; Piwowarczyk, W.; Pamula, E.; Liskova, J.; Schaubroeck, D.; Leeuwenburgh, S.C.; Brackman, G.; Balcaen, L.; Detsch, R.; Declercq, H.; et al. Injectable self-gelling composites for bone tissue engineering based on gellan gum hydrogel enriched with different bioglasses. Biomed. Mater. 2014, 9, 045014. [Google Scholar] [CrossRef] [PubMed]
- Douglas, T.E.L.; Krawczyk, G.; Pamula, E.; Declercq, H.A.; Schaubroeck, D.; Bucko, M.M.; Balcaen, L.; Van Der Voort, P.; Bliznuk, V.; van den Vreken, N.M.F.; et al. Generation of composites for bone tissue-engineering applications consisting of gellan gum hydrogels mineralized with calcium and magnesium phosphate phases by enzymatic means. J. Tissue Eng. Regener. Med. 2016, 10, 938–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, W.K.; Choi, J.H.; Shin, M.E.; Kim, J.W.; Kim, P.Y.; Kim, N.; Song, J.E.; Khang, G. Evaluation of cartilage regeneration of chondrocyte encapsulated gellan gum-based hyaluronic acid blended hydrogel. Int. J. Biol. Macromol. 2019, 141, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Thangavelu, M.; Cheolui, S.; Kim, H.S.; Choi, M.J.; Song, J.E.; Khang, G. Effect of different concentration of demineralized bone powder with gellan gum porous scaffold for the application of bone tissue regeneration. Int. J. Biol. Macromol. 2019, 134, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, D.; Jeong, Y.W.; Choi, M.J.; Lee, G.W.; Thangavelu, M.; Song, J.E.; Khang, G. Engineering retinal pigment epithelial cells regeneration for transplantation in regenerative medicine using PEG/Gellan gum hydrogels. Int. J. Biol. Macromol. 2019, 130, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.Y.; Park, J.H.; Shin, M.E.; Song, J.E.; Carlomagno, C.; Khang, G. Evaluation of Chondrogenic Differentiation Ability of Bone Marrow Mesenchymal Stem Cells in Silk Fibroin/Gellan Gum Hydrogels Using miR-30. Macromol. Res. 2019, 27, 369–376. [Google Scholar] [CrossRef]
- Baek, J.S.; Carlomagno, C.; Muthukumar, T.; Kim, D.; Park, J.H.; Song, J.E.; Migliaresi, C.; Motta, A.; Reis, R.L.; Khang, G. Evaluation of Cartilage Regeneration in Gellan Gum/agar Blended Hydrogel with Improved Injectability. Macromol. Res. 2019, 27, 558–564. [Google Scholar] [CrossRef]
- Jeon, H.Y.; Shin, E.Y.; Choi, J.H.; Song, J.E.; Reis, R.L.; Khang, G. Evaluation of Saponin Loaded Gellan Gum Hydrogel Scaffold for Cartilage Regeneration. Macromol. Res. 2018, 26, 724–729. [Google Scholar] [CrossRef] [Green Version]
- Choi, I.; Kim, C.; Song, J.E.; Baek, J.; Jeon, S.H.; Jeon, H.; Lee, S.Y.; Khang, G. A Comprehensive Study on Cartilage Regeneration Using Gellan-gum/Chondroitin Sulfate Hybrid Hydrogels. Polymer Korea 2017, 41, 962–966. [Google Scholar] [CrossRef]
- Jana, S.; Sen, K.K. Gellan gum/PVA Interpenetrating Network Micro-beads for Sustained Drug Delivery. Mater. Today-Proce. 2019, 11, 614–619. [Google Scholar] [CrossRef]
- Yu, I.; Kaonis, S.; Chen, R. A Study on Degradation Behavior of 3D Printed Gellan Gum Scaffolds. Procedia CIRP 2017, 65, 78–83. [Google Scholar] [CrossRef]
- Picone, C.S.F.; da Cunha, R.L. Interactions between milk proteins and gellan gum in acidified gels. Food Hydrocoll. 2010, 24, 502–511. [Google Scholar] [CrossRef]
- Karthika, J.S.; Vishalakshi, B.; Naik, J. Gellan gum-graft-polyaniline—An electrical conducting biopolymer. Int. J. Biol. Macromol. 2016, 82, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Novac, O.; Lisa, G.; Profire, L.; Tuchilus, C.; Popa, M.I. Antibacterial quaternized gellan gum based particles for controlled release of ciprofloxacin with potential dermal applications. Mat. Sci. Eng. C-Mater. 2014, 35, 291–299. [Google Scholar] [CrossRef]
- Kumar, S.; Kaur, P.; Bernela, M.; Rani, R.; Thakur, R. Ketoconazole encapsulated in chitosan-gellan gum nanocomplexes exhibits prolonged antifungal activity. Int. J. Biol. Macromol. 2016, 93, 988–994. [Google Scholar] [CrossRef]
- Sarkar, D.; Nandi, G.; Changder, A.; Hudati, P.; Sarkar, S.; Ghosh, L.K. Sustained release gastroretentive tablet of metformin hydrochloride based on poly (acrylic acid)-grafted-gellan. Int. J. Biol. Macromol. 2017, 96, 137–148. [Google Scholar] [CrossRef]
- Pacelli, S.; Paolicelli, P.; Moretti, G.; Petralito, S.; Di Giacomo, S.; Vitalone, A.; Casadei, M.A. Gellan gum methacrylate and laponite as an innovative nanocomposite hydrogel for biomedical applications. Eur. Polym. J. 2016, 77, 114–123. [Google Scholar] [CrossRef]
- Kulkarni, R.V.; Mangond, B.S.; Mutalik, S.; Sa, B. Interpenetrating polymer network microcapsules of gellan gum and egg albumin entrapped with diltiazem–resin complex for controlled release application. Carbohydr. Polym. 2011, 83, 1001–1007. [Google Scholar] [CrossRef]
- Oliveira, M.B.; Custodio, C.A.; Gasperini, L.; Reis, R.L.; Mano, J.F. Autonomous osteogenic differentiation of hASCs encapsulated in methacrylated gellan-gum hydrogels. Acta Biomater 2016, 41, 119–132. [Google Scholar] [CrossRef]
- Coutinho, D.F.; Sant, S.V.; Shin, H.; Oliveira, J.T.; Gomes, M.E.; Neves, N.M.; Khademhosseini, A.; Reis, R.L. Modified Gellan Gum hydrogels with tunable physical and mechanical properties. Biomaterials 2010, 31, 7494–7502. [Google Scholar] [CrossRef] [Green Version]
- Mazzuca, C.; Micheli, L.; Carbone, M.; Basoli, F.; Cervelli, E.; Iannuccelli, S.; Sotgiu, S.; Palleschi, A. Gellan hydrogel as a powerful tool in paper cleaning process: A detailed study. J. Colloid Interface Sci. 2014, 416, 205–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolan, L.C.; Matulka, R.A.; LeBeau, A.L.; Boulet, J.M. Two new nontoxic, non-pathogenic strains of Sphingomonas elodea for gellan gum production. Regul. Toxicol. Pharm. 2016, 78, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Matsukawa, S.; Tang, Z.; Watanabe, T. Hydrogen-bonding behavior of gellan in solution during structural change observed by 1H NMR and circular dichroism methods. In Physical Chemistry and Industrial Application of Gellan Gum; Nishinari, K., Ed.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 15–24. [Google Scholar]
- Shin, H.; Olsen, B.D.; Khademhosseini, A. The mechanical properties and cytotoxicity of cell-laden double-network hydrogels based on photocrosslinkable gelatin and gellan gum biomacromolecules. Biomaterials 2012, 33, 3143–3152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef] [Green Version]
- Silva-Correia, J.; Oliveira, J.M.; Caridade, S.G.; Oliveira, J.T.; Sousa, R.A.; Mano, J.F.; Reis, R.L. Gellan gum-based hydrogels for intervertebral disc tissue-engineering applications. J. Tissue Eng. Regener. Med. 2011, 5, e97–e107. [Google Scholar] [CrossRef] [Green Version]
- Ferris, C.J.; Stevens, L.R.; Gilmore, K.J.; Mume, E.; Greguric, I.; Kirchmajer, D.M.; Wallace, G.G.; in het Panhuis, M. Peptide modification of purified gellan gum. J. Mater. Chem. B 2015, 3, 1106–1115. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Sun, J.; Fan, H.; Zhang, X. An improved complex gel of modified gellan gum and carboxymethyl chitosan for chondrocytes encapsulation. Carbohydr. Polym. 2012, 88, 46–53. [Google Scholar] [CrossRef]
- Hahn, S.K.; Park, J.K.; Tomimatsu, T.; Shimoboji, T. Synthesis and degradation test of hyaluronic acid hydrogels. Int. J. Biol. Macromol. 2007, 40, 374–380. [Google Scholar] [CrossRef]
- Oudshoorn, M.H.M.; Rissmann, R.; Bouwstra, J.A.; Hennink, W.E. Synthesis of methacrylated hyaluronic acid with tailored degree of substitution. Polymer 2007, 48, 1915–1920. [Google Scholar] [CrossRef]
- Van Den Bulcke, A.I.; Bogdanov, B.; De Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules 2000, 1, 31–38. [Google Scholar] [CrossRef]
- Chou, A.I.; Nicoll, S.B. Characterization of photocrosslinked alginate hydrogels for nucleus pulposus cell encapsulation. J. Biomed. Mater. Res. 2009, 91, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Choi, O.K.; Lee, J.; Noh, J.; Lee, S.; Park, A.; Rim, M.A.; Reis, R.L.; Khang, G. Evaluation of double network hydrogel of poloxamer-heparin/gellan gum for bone marrow stem cells delivery carrier. Colloids Surf. B-Biointer. 2019, 181, 879–889. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Shah, P.A.; Shrivastav, P.S. Guar gum: Versatile natural polymer for drug delivery applications. Eur. Polym. J. 2019, 112, 722–735. [Google Scholar] [CrossRef]
- da Silva, L.P.; Cerqueira, M.T.; Sousa, R.A.; Reis, R.L.; Correlo, V.M.; Marques, A.P. Engineering cell-adhesive gellan gum spongy-like hydrogels for regenerative medicine purposes. Acta Biomater 2014, 10, 4787–4797. [Google Scholar] [CrossRef]
- Miyamoto, K.; Tsuji, K.; Nakamura, T.; Tokita, M.; Komai, T. Preparation of carboxymethyl-gellan. Carbohydr. Polym. 1996, 30, 161–164. [Google Scholar] [CrossRef]
- Du, H.; Hamilton, P.; Reilly, M.; Ravi, N. Injectable in situ physically and chemically crosslinkable gellan hydrogel. Macromol. Biosci. 2012, 12, 952–961. [Google Scholar] [CrossRef]
- D’Arrigo, G.; Navarro, G.; Di Meo, C.; Matricardi, P.; Torchilin, V. Gellan gum nanohydrogel containing anti-inflammatory and anti-cancer drugs: A multi-drug delivery system for a combination therapy in cancer treatment. Eur. J. Pharm. Biopharem. 2014, 87, 208–216. [Google Scholar] [CrossRef]
- Monica, R.P.R.; Mayuri, K.M. Multiparticulate Drug Delivery System for Gastrointestinal Tuberculosis. Int.J. Pharm. Sci. Drug Res. 2019, 11, 210–220. [Google Scholar]
- Tripathi, G.K.; Singh, S.; Nath, G. Formulation and In-vitro Evaluation of pH-Sensitive Oil Entrapped Polymeric Blend Amoxicillin Beads for the Eradication of Helicobacter pylori. Iran. J. Pharm. Res. 2012, 11, 447–455. [Google Scholar]
- Srinatha, A.; Pandit, J.K. Multi-unit floating alginate system: Effect of additives on ciprofloxacin release. Drug Deliv. 2008, 15, 471–476. [Google Scholar] [CrossRef]
- Milivojevic, M.; Pajic-Lijakovic, I.; Bugarski, B.; Nayak, A.K.; Hasnain, M.S. Chapter 6-Gellan gum in drug delivery applications. In Natural Polysaccharides in Drug Delivery and Biomedical Applications; Hasnain, M.S., Nayak, A.K., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 145–186. [Google Scholar]
- Dhar, S.; Mali, V.; Bodhankar, S.; Shiras, A.; Prasad, B.L.; Pokharkar, V. Biocompatible gellan gum-reduced gold nanoparticles: Cellular uptake and subacute oral toxicity studies. J. Appl. Toxic. 2011, 31, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Reddy, E.M.; Shiras, A.; Pokharkar, V.; Prasad, B.L. Natural gum reduced/stabilized gold nanoparticles for drug delivery formulations. Chemistry (Weinheim an der Bergstrasse, Germany) 2008, 14, 10244–10250. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Murawala, P.; Shiras, A.; Pokharkar, V.; Prasad, B.L. Gellan gum capped silver nanoparticle dispersions and hydrogels: Cytotoxicity and in vitro diffusion studies. Nanoscale 2012, 4, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Vieira, S.; Vial, S.; Maia, F.R.; Carvalho, M.; Reis, R.L.; Granja, P.L.; Oliveira, J.M. Gellan gum-coated gold nanorods: An intracellular nanosystem for bone tissue engineering. RSC Adv. 2015, 5, 77996–78005. [Google Scholar] [CrossRef]
- Fialho, A.M.; Moreira, L.M.; Granja, A.T.; Popescu, A.O.; Hoffmann, K.; Sa-Correia, I. Occurrence, production, and applications of gellan: Current state and perspectives. Appl. Microbiol. Biotechnol. 2008, 79, 889–900. [Google Scholar] [CrossRef]
- Rozier, A.; Mazuel, C.; Grove, J.; Plazonnet, B. Gelrite®: A novel, ion-activated, in-situ gelling polymer for ophthalmic vehicles. Effect on bioavailability of timolol. Int. J. Pharm. 1989, 57, 163–168. [Google Scholar] [CrossRef]
- Gal, A.; Nussinovitch, A. Hydrocolloid carriers with filler inclusion for diltiazem hydrochloride release. J. Pharm. Sci. 2007, 96, 168–178. [Google Scholar] [CrossRef]
- Li, J.; Kamath, K.; Dwivedi, C. Gellan film as an implant for insulin delivery. J. Biomater. Appl. 2001, 15, 321–343. [Google Scholar] [CrossRef]
- Jeon, S.H.; Lee, W.T.; Song, J.E.; Park, H.; Choi, I.N.; Kim, C.M.; Khang, G. Cartilage Regeneration Using Hesperidin-Containing Gellan Gum Scaffolds. Polym. Korea 2017, 41, 670–674. [Google Scholar] [CrossRef]
- Bhalerao, H.; Koteshwara, K.B.; Chandran, S. Levofloxacin Hemihydrate In Situ Gelling Ophthalmic Solution: Formulation Optimization and In Vitro and In Vivo Evaluation. AAPS Pharm. Sci. Tech. 2019, 20, 272. [Google Scholar] [CrossRef]
- Vashisth, P.; Raghuwanshi, N.; Srivastava, A.K.; Singh, H.; Nagar, H.; Pruthi, V. Ofloxacin loaded gellan/PVA nanofibers - Synthesis, characterization and evaluation of their gastroretentive/mucoadhesive drug delivery potential. Mater. Sci. Eng-C 2017, 71, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Vashisth, P.; Nikhil, K.; Roy, P.; Pruthi, P.A.; Singh, R.P.; Pruthi, V. A novel gellan–PVA nanofibrous scaffold for skin tissue regeneration: Fabrication and characterization. Carbohydr. Polym. 2016, 136, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Rostami, M.; Ghorbani, M.; Aman mohammadi, M.; Delavar, M.; Tabibiazar, M.; Ramezani, S. Development of resveratrol loaded chitosan-gellan nanofiber as a novel gastrointestinal delivery system. Int. J. Biol. Macromol. 2019, 135, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Mehnath, S.; Ayisha Sithika, M.A.; Arjama, M.; Rajan, M.; Amarnath Praphakar, R.; Jeyaraj, M. Sericin-chitosan doped maleate gellan gum nanocomposites for effective cell damage in Mycobacterium tuberculosis. Int. J. Biol. Macromol. 2019, 122, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Tiwari, A.; Panda, P.K.; Saraf, S.; Jain, A.; Jain, S.K. Chapter 8-Locust bean gum in drug delivery application. In Natural Polysaccharides in Drug Delivery and Biomedical Applications; Hasnain, M.S., Nayak, A.K., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 203–222. [Google Scholar]
- Izawa, H.; Nishino, S.; Maeda, H.; Morita, K.; Ifuku, S.; Morimoto, M.; Saimoto, H.; Kadokawa, J.-i. Mineralization of hydroxyapatite upon a unique xanthan gum hydrogel by an alternate soaking process. Carbohydr. Polym. 2014, 102, 846–851. [Google Scholar] [CrossRef] [Green Version]
- Kuo, S.M.; Chang, S.J.; Wang, H.Y.; Tang, S.C.; Yang, S.W. Evaluation of the ability of xanthan gum/gellan gum/hyaluronan hydrogel membranes to prevent the adhesion of postrepaired tendons. Carbohydr. Polym. 2014, 114, 230–237. [Google Scholar] [CrossRef]
- Oliveira Cardoso, V.M.; Stringhetti Ferreira Cury, B.; Evangelista, R.C.; Daflon Gremiao, M.P. Development and characterization of cross-linked gellan gum and retrograded starch blend hydrogels for drug delivery applications. J. Mech Behav Biomed. Mater. 2017, 65, 317–333. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Ferreiro, A.; Silva-Rodriguez, J.; Otero-Espinar, F.J.; Gonzalez-Barcia, M.; Lamas, M.J.; Ruibal, A.; Luaces-Rodriguez, A.; Vieites-Prado, A.; Lema, I.; Herranz, M.; et al. In vivo eye surface residence determination by high-resolution scintigraphy of a novel ion-sensitive hydrogel based on gellan gum and kappa-carrageenan. Eur. J. Pharm. Biopharem. 2017, 114, 317–323. [Google Scholar] [CrossRef]
- Emeje, M.O.; Franklin-Ude, P.I.; Ofoefule, S.I. Evaluation of the fluid uptake kinetics and drug release from gellan gum tablets containing metronidazole. Int. J. Biol. Macromol. 2010, 47, 158–163. [Google Scholar] [CrossRef]
- Qin, F.; Man, J.; Cai, C.; Xu, B.; Gu, M.; Zhu, L.; Shi, Y.-C.; Liu, Q.; Wei, C. Physicochemical properties of high-amylose rice starches during kernel development. Carbohydr. Polym. 2012, 88, 690–698. [Google Scholar] [CrossRef]
- Rajinikanth, P.S.; Mishra, B. Preparation and in vitro characterization of gellan based floating beads of acetohydroxamic acid for eradication of H. pylori. Acta Pharm. (Zagreb, Croatia) 2007, 57, 413–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiavi, A.; Cuccaro, R.; Troia, A. Strain-rate and temperature dependent material properties of Agar and Gellan Gum used in biomedical applications. J. Mech Behav Biomed. Mater. 2016, 53, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Amin, K.A.M.; Panhuis, M.i.h. Polyelectrolyte complex materials from chitosan and gellan gum. Carbohydr. Polym. 2011, 86, 352–358. [Google Scholar] [CrossRef] [Green Version]
- Ahuja, M.; Yadav, M.; Kumar, S. Application of response surface methodology to formulation of ionotropically gelled gum cordia/gellan beads. Carbohydr. Polym. 2010, 80, 161–167. [Google Scholar] [CrossRef]
- Ismail, N.A.; Amin, K.A.M.; Majid, F.A.A.; Razali, M.H. Gellan gum incorporating titanium dioxide nanoparticles biofilm as wound dressing: Physicochemical, mechanical, antibacterial properties and wound healing studies. Mat. Sci. Eng. C-Mater. 2019, 103, 109770. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L.R.; Gilmore, K.J.; Wallace, G.G.; in het Panhuis, M. Tissue engineering with gellan gum. Biomater. Sci. 2016, 4, 1276–1290. [Google Scholar] [CrossRef] [Green Version]
- Cerqueira, M.T.; da Silva, L.P.; Santos, T.C.; Pirraco, R.P.; Correlo, V.M.; Reis, R.L.; Marques, A.P. Gellan gum-hyaluronic acid spongy-like hydrogels and cells from adipose tissue synergize promoting neoskin vascularization. ACS Appl Mater. Interfaces 2014, 6, 19668–19679. [Google Scholar] [CrossRef]
- Kirchmajer, D.M.; Steinhoff, B.; Warren, H.; Clark, R.; in het Panhuis, M. Enhanced gelation properties of purified gellan gum. Carbohydr Res. 2014, 388, 125–129. [Google Scholar] [CrossRef] [Green Version]
- Pina, S.; Oliveira, J.M.; Reis, R.L. Natural-based nanocomposites for bone tissue engineering and regenerative medicine: A review. Advanced Mater. (Deerfield Beach, Fla.) 2015, 27, 1143–1169. [Google Scholar] [CrossRef] [Green Version]
- Douglas, T.E.; Wlodarczyk, M.; Pamula, E.; Declercq, H.A.; de Mulder, E.L.; Bucko, M.M.; Balcaen, L.; Vanhaecke, F.; Cornelissen, R.; Dubruel, P.; et al. Enzymatic mineralization of gellan gum hydrogel for bone tissue-engineering applications and its enhancement by polydopamine. J. Tissue Eng. Regener. Med. 2014, 8, 906–918. [Google Scholar] [CrossRef]
- Khang, G.; Lee, S.K.; Kim, H.N.; Silva-Correia, J.; Gomes, M.E.; Viegas, C.A.; Dias, I.R.; Oliveira, J.M.; Reis, R.L. Biological evaluation of intervertebral disc cells in different formulations of gellan gum-based hydrogels. J. Tissue Eng. Regener. Med. 2015, 9, 265–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, H.; Olsen, B.D.; Khademhosseini, A. Gellan gum microgel-reinforced cell-laden gelatin hydrogels. J. Mater. Chem. B 2014, 2, 2508–2516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Li, Z.; Jiang, S.; Bratlie, K.M. Chemically Modified Gellan Gum Hydrogels with Tunable Properties for Use as Tissue Engineering Scaffolds. ACS Omega 2018, 3, 6998–7007. [Google Scholar] [CrossRef] [PubMed]
- Vilela, C.A.; Correia, C.; da Silva Morais, A.; Santos, T.C.; Gertrudes, A.C.; Moreira, E.S.; Frias, A.M.; Learmonth, D.A.; Oliveira, P.; Oliveira, J.M.; et al. In vitro and in vivo performance of methacrylated gellan gum hydrogel formulations for cartilage repair. J. Biomed. Mater. Res. Part A 2018, 106, 1987–1996. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.W.; Tsai, H.F.; Wen, S.M.; Huang, C.H. Photocrosslinkable gellan gum film as an anti-adhesion barrier. Carbohydr. Polym. 2012, 90, 1132–1138. [Google Scholar] [CrossRef]
- Visser, J.; Peters, B.; Burger, T.J.; Boomstra, J.; Dhert, W.J.; Melchels, F.P.; Malda, J. Biofabrication of multi-material anatomically shaped tissue constructs. Biofabrication 2013, 5, 035007. [Google Scholar] [CrossRef]
- Adrover, A.; Paolicelli, P.; Petralito, S.; Di Muzio, L.; Trilli, J.; Cesa, S.; Tho, I.; Casadei, M.A. Gellan Gum/Laponite Beads for the Modified Release of Drugs: Experimental and Modeling Study of Gastrointestinal Release. Pharmaceutics 2019, 11, 187. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Wang, Z. A pH-sensitive microemulsion-filled gellan gum hydrogel encapsulated apigenin: Characterization and in vitro release kinetics. Colloids Surf. B Biointerfaces 2019, 178, 245–252. [Google Scholar] [CrossRef]
- Gering, C.; Koivisto, J.T.; Parraga, J.; Leppiniemi, J.; Vuornos, K.; Hytonen, V.P.; Miettinen, S.; Kellomaki, M. Design of modular gellan gum hydrogel functionalized with avidin and biotinylated adhesive ligands for cell culture applications. PloS ONE 2019, 14, e0221931. [Google Scholar] [CrossRef] [Green Version]
- Bastos, A.R.; da Silva, L.P.; Maia, F.R.; Pina, S.; Rodrigues, T.; Sousa, F.; Oliveira, J.M.; Cornish, J.; Correlo, V.M.; Reis, R.L. Lactoferrin-Hydroxyapatite Containing Spongy-Like Hydrogels for Bone Tissue Engineering. Materials (Basel) 2019, 12, 2074. [Google Scholar] [CrossRef] [Green Version]
- D’Arrigo, G.; Di Meo, C.; Gaucci, E.; Chichiarelli, S.; Coviello, T.; Capitani, D.; Alhaique, F.; Matricardi, P. Self-assembled gellan-based nanohydrogels as a tool for prednisolone delivery. Soft Matter 2012, 8, 11557–11564. [Google Scholar] [CrossRef]
- Swain, G.P.; Patel, S.; Gandhi, J.; Shah, P. Development of Moxifloxacin Hydrochloride loaded in-situ gel for the treatment of periodontitis: In-vitro drug release study and antibacterial activity. J. Oral Biol. Craniofac. Res. 2019, 9, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Aadil, K.R.; Nathani, A.; Sharma, C.S.; Lenka, N.; Gupta, P. Investigation of poly(vinyl) alcohol-gellan gum based nanofiber as scaffolds for tissue engineering applications. J. Drug Deliv. Sci. Technol. 2019, 54, 101276. [Google Scholar] [CrossRef]
- Barbosa, E.J.; Ferraz, H.G. Gellan gum and polyvinylpyrrolidone (PVP) as binding agents in extrusion/spheronization pellet formulations. Acta Pharm. (Zagreb, Croatia) 2019, 69, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Gong, Y.; Lin, Y.; Shen, J.; Wang, D.A. A novel gellan gel-based microcarrier for anchorage-dependent cell delivery. Acta Biomater 2008, 4, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Rada, T.; Carvalho, P.P.; Santos, T.C.; Castro, A.G.; Reis, R.L.; Gomes, M.E. Chondrogenic potential of two hASCs subpopulations loaded onto gellan gum hydrogel evaluated in a nude mice model. Curr. Stem. Cell Res. Ther. 2013, 8, 357–364. [Google Scholar] [CrossRef]
- Ahearne, M.; Kelly, D.J. A comparison of fibrin, agarose and gellan gum hydrogels as carriers of stem cells and growth factor delivery microspheres for cartilage regeneration. Biomed. Mater. (Bristol, England) 2013, 8, 035004. [Google Scholar] [CrossRef]
- Duarte Pereira, H.M.; Silva-Correia, J.; Yan, L.-P.; Caridade, S.G.; Frias, A.M.; Oliveira, A.L.; Mano, J.F.; Oliveira, J.M.; Espregueira-Mendes, J.; dos Reis, R.L.G.; et al. Silk-Fibroin/Methacrylated Gellan Gum Hydrogel As An Novel Scaffold For Application In Meniscus Cell-Based Tissue Engineering. Arthroscopy 2013, 29, e53–e55. [Google Scholar] [CrossRef]
- Ku, K.C.; Lee, M.W.; Kuo, S.M.; Yao, C.H.; Chang, S.J. Preparation and evaluation of collagen I/ gellan gum/beta-TCP microspheres as bone graft substitute materials. In Proceedings of the 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Osaka, Japan, 3–7 July 2013; pp. 6667–6670. [Google Scholar]
- Silva-Correia, J.; Gloria, A.; Oliveira, M.B.; Mano, J.F.; Oliveira, J.M.; Ambrosio, L.; Reis, R.L. Rheological and mechanical properties of acellular and cell-laden methacrylated gellan gum hydrogels. J. Biomed. Mater. Res. 2013, 101, 3438–3446. [Google Scholar] [CrossRef] [Green Version]
- Mouser, V.H.; Melchels, F.P.; Visser, J.; Dhert, W.J.; Gawlitta, D.; Malda, J. Yield stress determines bioprintability of hydrogels based on gelatin-methacryloyl and gellan gum for cartilage bioprinting. Biofabrication 2016, 8, 035003. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Dhert, W.J.A.; Hutmacher, D.W.; Malda, J. Development and characterisation of a new bioink for additive tissue manufacturing. J. Mater. Chem. B 2014, 2, 2282–2289. [Google Scholar] [CrossRef] [Green Version]
- Vijan, V.; Kaity, S.; Biswas, S.; Isaac, J.; Ghosh, A. Microwave assisted synthesis and characterization of acrylamide grafted gellan, application in drug delivery. Carbohydr. Polym. 2012, 90, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Osmalek, T.Z.; Froelich, A.; Sobol, M.; Milanowski, B.; Skotnicki, M.; Kunstman, P.; Szybowicz, M. Gellan gum macrobeads loaded with naproxen: The impact of various naturally derived polymers on pH-dependent behavior. J. Biomater. Appl. 2018, 33, 140–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agnihotri, S.A.; Aminabhavi, T.M. Development of novel interpenetrating network gellan gum-poly(vinyl alcohol) hydrogel microspheres for the controlled release of carvedilol. Drug Develop. Industr. Pharm. 2005, 31, 491–503. [Google Scholar] [CrossRef]
- Wu, R.L.; Zhao, C.S.; Xie, J.W.; Yi, S.L.; Song, H.T.; He, Z.G. Preparation of in situ gel systems for the oral delivery of ibuprofen and its pharmacokinetics study in beagle dogs. Acta pharmac. Sinica 2008, 43, 956–962. [Google Scholar]
- Bhattacharya, S.S.; Banerjee, S.; Chowdhury, P.; Ghosh, A.; Hegde, R.R.; Mondal, R. Tranexamic acid loaded gellan gum-based polymeric microbeads for controlled release: In vitro and in vivo assessment. Colloids Surf. B Biointerfaces 2013, 112, 483–491. [Google Scholar] [CrossRef]
- Cencetti, C.; Bellini, D.; Pavesio, A.; Senigaglia, D.; Passariello, C.; Virga, A.; Matricardi, P. Preparation and characterization of antimicrobial wound dressings based on silver, gellan, PVA and borax. Carbohydr. Polym. 2012, 90, 1362–1370. [Google Scholar] [CrossRef]
- Shukla, R.; Kashaw, S.K.; Jain, A.P.; Lodhi, S. Fabrication of Apigenin loaded gellan gum-chitosan hydrogels (GGCH-HGs) for effective diabetic wound healing. Int. J. Biol. Macromol. 2016, 91, 1110–1119. [Google Scholar] [CrossRef]
- Tsai, W.; Tsai, H.; Wong, Y.; Hong, J.; Chang, S.; Lee, M. Preparation and characterization of gellan gum/glucosamine/clioquinol film as oral cancer treatment patch. Mat. Sci. Eng. C-Mater. 2018, 82, 317–322. [Google Scholar] [CrossRef]
- Song, J.E.; Song, Y.S.; Jeon, S.H.; Choi, I.N.; Kim, C.M.; Khang, G. Evaluation of Gelatin and Gellan Gum Blended Hydrogel for Cartilage Regeneration. Polymer Korea 2017, 41, 619–623. [Google Scholar] [CrossRef]
- Song, J.E.; Lee, S.E.; Cha, S.R.; Jang, N.K.; Tripathy, N.; Reis, R.L.; Khang, G. Inflammatory response study of gellan gum impregnated duck’s feet derived collagen sponges. J. Biomater. Sci. Polym. Ed. 2016, 27, 1495–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vashisth, P.; Pruthi, P.A.; Singh, R.P.; Pruthi, V. Process optimization for fabrication of gellan based electrospun nanofibers. Carbohydr. Polym. 2014, 109, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Bera, H.; Ang, S.R.; Chiong, S.W.; Chan, C.H.; Abbasi, Y.F.; Law, L.P.; Chatterjee, B.; Venugopal, V. Core-shell structured pullulan based nanocomposites as erlotinib delivery shuttles. Int. J. Polym. Mater. Polym. Biomater. 2019, 1–12. [Google Scholar] [CrossRef]
- Tatke, A.; Dudhipala, N.; Janga, K.Y.; Balguri, S.P.; Avula, B.; Jablonski, M.M.; Majumdar, S. In Situ Gel of Triamcinolone Acetonide-Loaded Solid Lipid Nanoparticles for Improved Topical Ocular Delivery: Tear Kinetics and Ocular Disposition Studies. Nanomaterials (Basel, Switzerland) 2018, 9, 33. [Google Scholar] [CrossRef] [Green Version]
- Arjama, M.; Mehnath, S.; Rajan, M.; Jeyaraj, M. Sericin/RBA embedded gellan gum based smart nanosystem for pH responsive drug delivery. Int. J. Biol. Macromol. 2018, 120, 1561–1571. [Google Scholar] [CrossRef]
- Janga, K.Y.; Tatke, A.; Balguri, S.P.; Lamichanne, S.P.; Ibrahim, M.M.; Maria, D.N.; Jablonski, M.M.; Majumdar, S. Ion-sensitive in situ hydrogels of natamycin bilosomes for enhanced and prolonged ocular pharmacotherapy: In vitro permeability, cytotoxicity and in vivo evaluation. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1039–1050. [Google Scholar] [CrossRef] [Green Version]
- Dhanka, M.; Shetty, C.; Srivastava, R. Methotrexate loaded gellan gum microparticles for drug delivery. Int. J. Biol. Macromol. 2018, 110, 346–356. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, Z. A novel ocular delivery of brinzolamide based on gellan gum: In vitro and in vivo evaluation. Drug Des. Devel. Ther. 2018, 12, 383–389. [Google Scholar] [CrossRef] [Green Version]
- Silva-Correia, J.; Miranda-Goncalves, V.; Salgado, A.J.; Sousa, N.; Oliveira, J.M.; Reis, R.M.; Reis, R.L. Angiogenic potential of gellan-gum-based hydrogels for application in nucleus pulposus regeneration: In vivo study. Tissue Eng. Part. A 2012, 18, 1203–1212. [Google Scholar] [CrossRef] [Green Version]
- Ayala, R.; Zhang, C.; Yang, D.; Hwang, Y.; Aung, A.; Shroff, S.S.; Arce, F.T.; Lal, R.; Arya, G.; Varghese, S. Engineering the cell-material interface for controlling stem cell adhesion, migration, and differentiation. Biomaterials 2011, 32, 3700–3711. [Google Scholar] [CrossRef]
- Annabi, N.; Nichol, J.W.; Zhong, X.; Ji, C.; Koshy, S.; Khademhosseini, A.; Dehghani, F. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng. Part. B Rev. 2010, 16, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Frey, M.T.; Wang, Y.L. A photo-modulatable material for probing cellular responses to substrate rigidity. Soft Matter 2009, 5, 1918–1924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauswirth, W.W.; Aleman, T.S.; Kaushal, S.; Cideciyan, A.V.; Schwartz, S.B.; Wang, L.; Conlon, T.J.; Boye, S.L.; Flotte, T.R.; Byrne, B.J.; et al. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: Short-term results of a phase I trial. Hum. Gene Ther. 2008, 19, 979–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Jenwitheesuk, E.; Teller, D.C.; Samudrala, R. Structural insights into the cellular retinaldehyde-binding protein (CRALBP). Proteins 2005, 61, 412–422. [Google Scholar] [CrossRef]
- Amirpour, N.; Karamali, F.; Rabiee, F.; Rezaei, L.; Esfandiari, E.; Razavi, S.; Dehghani, A.; Razmju, H.; Nasr-Esfahani, M.H.; Baharvand, H. Differentiation of human embryonic stem cell-derived retinal progenitors into retinal cells by Sonic hedgehog and/or retinal pigmented epithelium and transplantation into the subretinal space of sodium iodate-injected rabbits. Stem Cells Develop. 2012, 21, 42–53. [Google Scholar] [CrossRef] [Green Version]










| Sl No | GG Composites | Applications | Reference |
|---|---|---|---|
| 1 | Xanthan gum (XG) -HAp | Bone tissue engineering | [90] |
| 2 | GG-XG-hyaluronan | Bone tissue engineering | [91] |
| 3 | GG/Starch | Drug delivery system | [92] |
| 4 | GG/alpha amylase | Pharmaceutical and biomedical | [5] |
| 5 | GG/PVA-Ofloxacin | Gastroretentive/mucoadhesive drug delivery | [85] |
| 6 | GG/kappa-carrageenan | Drugs on the ocular surface | [93] |
| 7 | GG/Chitosan | Nasal insert, antifungal agent, coatings, wound healing, antibiotic | [47,94,95,96] [14] |
| 8 | GG/kappa-carrageenan/alginates | Antifungal and antimicrobial drugs | [97] |
| 9 | GG/XG | Anti-adhesive | [98] |
| 10 | GG/pectin | Drug delivery | [15] |
| 11 | GG/agar | Biomedical applications | [99] |
| 12 | GG methacrylate/gelatin methacrylamide | In scaffolds for load-bearing tissues | [91] |
| 13 | GG/alginate | Sustained drug release | [16] |
| 14 | GG/titanium dioxide nanoparticles | Wound healing | [100] |
| 15 | GG/HAp | Bone, vasculature | [31,101] [102] |
| 16 | GG/Gelatin/genipin | material | [103] |
| 17 | GG/PLGA microsphere | Vertebra | [101] |
| 18 | GG/Gold nanorods | Bone | [104] |
| 19 | GG/Bioglass | Bone | [32,105] |
| 20 | GG/Graphine oxide | Scaffold | [106] |
| 21 | GG/HAGG/LAGG blends methacrylation/HA | Intervertebral discs | [106] |
| 22 | GG//methacrylation/GG microsphere/gelatin | Load bearing tissue | [107] |
| 23 | GG/methacrylation | Intervertebral discs, TE, cartilage repair | [58,108,109] |
| 24 | GG/Cinnamate | Wound healing | [110] |
| 25 | GG/Methacrylated gelatin | Cartilage | [111] |
| 26 | GG/HA | Skin repair/vascularization/cartilage regeneration | [102] [35] |
| 27 | GG/Laponite beads | Drug release | [112] |
| 28 | GG/ gum cordia | Drug delivery | [99] |
| 29 | GG/apigenin | Drug release | [113] |
| 30 | GG/avidin/boptinylated adhesive | Cell culture | [114] |
| 31 | GG/HAp/Lactoferrin | Bone tissue engineering | [115,116] |
| 32 | GG/AuNPs | Anti-cancer drug delivery | [75] |
| 33 | GG/AuNPs/DOX | Anti-cancer drug delivery | [76] |
| 34 | GG/AgNPs | Antibacterial, cytotoxic | [77] |
| 35 | GG/AuNRs | Intercellular drug delivery and imaging | [78] |
| 36 | GG/poloxamer 407/carbopol 934P) | Controlled delivery and antibacterial activity | [117] |
| 37 | GG/Lactoferrin | Bone Tissue Engineering | [115] |
| 38 | GG/insulin | Drug delivery | [82] |
| 39 | GG/poly(vinyl) alcohol | Tissue Engineering | [118] |
| 40 | GG/levofloxacin hemihydrate | Ophthalmic solution | [84] |
| 41 | GG/Polyvinylpyrrolidone (PVP) | Sustained release | [119] |
| 42 | Gelatin-grafted-GG-hydrogel microsphere | Cell encapsulation and delivery | [120] |
| 43 | GG hydrogel | Cartilage Tissue Engineering | [23], [121] |
| 43 | GG/fibrin/agarose | Cartilage regeneration | [122] |
| 44 | Ionic crosslinked methacrylated GG/Silk | Meniscus tissue engineering | [123] |
| 45 | GG/Polydopamine | Bone tissue engineering | [105] |
| 46 | GG/Collagen I, Beta -TCP | Bone graft material | [124] |
| 47 | GG-MA hydrogels | Intervertebral Disc | [28,106] [125] |
| 48 | GG/RGD | Cell adhesion, proliferation | [120] |
| 49 | GG/ UV crosslinked gelatin-methacryloyl (geMA) | Cartilage grafts bioprinting | [126,127] |
| 50 | GG/acrylamide grafted | Sustained release | [128] |
| 51 | GG/ dextran sulfates/ cellulose sulfate | Drug delivery | [129] |
| 52 | GG/polyvinylalcoho | Beta-blocker | [130] |
| 53 | GG/alginate | Antibiotic, Antinflammatory | [73,131] |
| 54 | GG/polyvinylalcohol | Antibiotic | [132] |
| 55 | GG/hyaluronic acid ester/polyvinylalcohol | Wound healing | [133] |
| 56 | GG/chitosan/PEG | Wound healing | [134] |
| 57 | GG/glucosamine | Oral cancer treatment | [135] |
| 58 | GG/ HA | Cartilage regeneration | [35] |
| 59 | GG/ poloxamer-heparin | Bone marrow stem cells delivery | [65] |
| 60 | GG/PEG | Retinal pigment epithelial cells regeneration | [37] |
| 61 | GG/ demineralized bone powder | Bone tissue regeneration | [36] |
| 62 | GG/Agar | Cartilage regeneration | [39] |
| 63 | GG/Silk fibroin | Chondrogenic differentiation | [38] |
| 64 | GG/Saponin | Cartilage regeneration | [40] |
| 65 | GG/Chondroitin sulfate | Cartilage regeneration | [41] |
| 66 | GG/ Gelatin | Cartilage regeneration | [136] |
| 67 | GG/Hesperidin | Cartilage regeneration | [83] |
| 68 | GG/ duck feet derived collagen | Tissue Engineering | [137] |
| 69 | GG hydrogel | Intervertebral disc | [106] |
| 70 | GG/ polyvinyl alcohol | Skin tissue regeneration | [86] |
| 71 | GG/PVA/Water | Skin tissue regeneration | [138] |
| 72 | GG/Chitosan/ resveratrol | Gastrointestinal delivery | [87] |
| 73 | GG/apigenin | Oral drug delivery | [113] |
| 74 | GG/Laponite Beads | Gastrointestinal drug release | [112] |
| 75 | Maleate GG/Sericin-chitosan | Mycobacterium tuberculosis | [88] |
| 76 | GG/sodium alginate/low-methoxyl pectin coated carboxymethyl pullulan-ZnO nanocomposites encapsulating erlotinib | Lung cancer therapy | [139] |
| 77 | GG/Triamcinolone acetonide | Topical Ocular Delivery | [140] |
| 78 | GG/Sericin/rice bran albumin | Cancer drug delivery | [141] |
| 79 | GG/natamycin bilosomes | Ocular pharmacotherapy | [142] |
| 80 | GG/Methotrexate | Drug delivery | [143] |
| 81 | GG/brinzolamide | Ocular delivery | [144] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muthukumar, T.; Song, J.E.; Khang, G. Biological Role of Gellan Gum in Improving Scaffold Drug Delivery, Cell Adhesion Properties for Tissue Engineering Applications. Molecules 2019, 24, 4514. https://doi.org/10.3390/molecules24244514
Muthukumar T, Song JE, Khang G. Biological Role of Gellan Gum in Improving Scaffold Drug Delivery, Cell Adhesion Properties for Tissue Engineering Applications. Molecules. 2019; 24(24):4514. https://doi.org/10.3390/molecules24244514
Chicago/Turabian StyleMuthukumar, Thangavelu, Jeong Eun Song, and Gilson Khang. 2019. "Biological Role of Gellan Gum in Improving Scaffold Drug Delivery, Cell Adhesion Properties for Tissue Engineering Applications" Molecules 24, no. 24: 4514. https://doi.org/10.3390/molecules24244514