In Situ Real-Time Tracing of Organophosphorus Pesticides in Apples by Solid-Phase Microextraction with Developed Sampling-Rate Calibration
Abstract
:1. Introduction
2. Results and Discussion
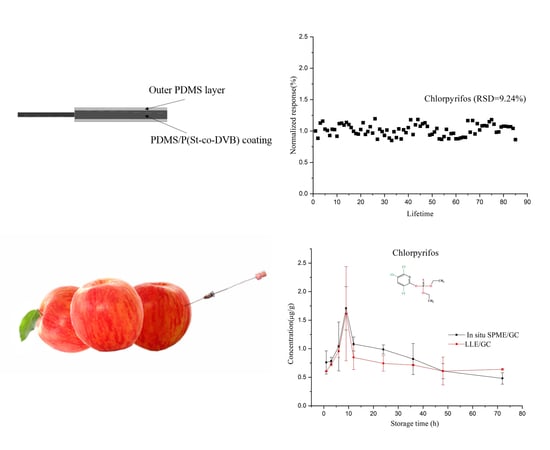
2.1. Strategies to Ensure the Lifetime of the Fiber in Apple Matrix
2.2. Stability of OPPs in Apple Matrix and Analytical Performance of the Fiber
2.3. Preparation of Positive Samples for In Situ Analysis
2.4. and Its Applicability
2.5. Evaluation of In Situ SPME/GC Method
2.6. In Situ Tracing OPPs in Apples
3. Materials and Methods
3.1. Chemical Reagents, Materials, and Samples
3.2. Instruments and Chromatographic Conditions
3.3. Preparation of SPME Fibers, SPME Procedure, LLE Procedure
3.4. In Situ SPME
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kou, X.; Chen, G.; Huang, S.; Ye, Y.; Ouyang, G.; Gan, J.; Zhu, F. In vivo sampling: A promising technique for detecting and profiling endogenous substances in living systems. J. Agric. Food Chem. 2019, 67, 2120–2126. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, G.; Huang, S.; Qiu, J.; Jiang, R.; Zhu, F.; Ouyang, G. Application of in vivo solid-phase microextraction in environmental analysis. TrAC Trends Anal. Chem. 2016, 85, 26–35. [Google Scholar] [CrossRef]
- Souza-Silva, E.A.; Jiang, R.; Rodríguez-Lafuente, A.; Gionfriddo, E.; Pawliszyn, J. A critical review of the state of the art of solid-phase microextraction of complex matrices I. Environmental analysis. TrAC Trends Anal. Chem. 2015, 71, 224–235. [Google Scholar] [CrossRef]
- Souza-Silva, E.A.; Gionfriddo, E.; Pawliszyn, J. A critical review of the state of the art of solid-phase microextraction of complex matrices II. Food analysis. TrAC Trends Anal. Chem. 2015, 71, 236–248. [Google Scholar] [CrossRef]
- Souza-Silva, E.A.; Reyes-Garces, N.; Gomez-Ríos, G.A.; Boyaci, E.; Bojko, B.; Pawliszyn, J. A critical review of the state of the art of solid-phase microextraction of complex matrices III. Bioanalytical and clinical applications. TrAC Trends Anal. Chem. 2015, 71, 249–264. [Google Scholar] [CrossRef]
- Qiu, J.; Chen, G.; Xu, J.; Luo, E.; Liu, Y.; Wang, F.; Zhou, H.; Liu, Y.; Zhu, F.; Ouyang, G. In vivo tracing of organochloride and organophosphorus pesticides in different organs of hydroponically grown malabar spinach (Basella alba L.). J. Hazard. Mater. 2016, 316, 52–59. [Google Scholar] [CrossRef]
- Qiu, J.; Chen, G.; Zhou, H.; Xu, J.; Wang, F.; Zhu, F.; Ouyang, G. In vivo tracing of organophosphorus pesticides in cabbage (Brassica parachinensis) and aloe (Barbadensis). Sci. Total Environ. 2016, 550, 1134–1140. [Google Scholar] [CrossRef]
- Shi, Z.; Chen, D.; Chen, T.; Wei, G.; Yin, C.; Xu, H.; Yang, G. In vivo analysis of two new fungicides in mung bean sprouts by solid-phase microextraction-gas chromatography-mass spectrometry. Food Chem. 2019, 275, 688–695. [Google Scholar] [CrossRef]
- Qiu, J.; Chen, G.; Liu, S.; Zhang, T.; Wu, J.; Wang, F.; Xu, J.; Liu, Y.; Zhu, F.; Ouyang, G. Bioinspired polyelectrolyte-assembled graphene-oxide-coated C18 composite solid-phase microextraction fibers for in vivo monitoring of acidic pharmaceuticals in fish. Anal. Chem. 2016, 88, 5841–5848. [Google Scholar] [CrossRef]
- Wang, D.; Gao, D.; Huang, Y.; Xu, W.; Xia, Z. Preparation of restricted access molecularly imprinted polymers based fiber for selective solid-phase microextraction of hesperetin and its metabolites in vivo. Talanta 2019, 202, 392–401. [Google Scholar] [CrossRef]
- Lendor, S.; Hassani, S.A.; Boyaci, E.; Singh, V.; Womelsdorf, T.; Pawliszyn, J. Solid-phase microextraction-based miniaturized probe and protocol for extraction of neurotransmitters from brains in vivo. Anal. Chem. 2019, 91, 4896–4905. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Li, W.; Yang, Q.; Liu, Y.; Fang, L.; Guo, Y.; Guo, P.; Lin, L.; Yang, Y.; Luan, T. Biocompatible surface-coated probe for in vivo, in situ, and microscale lipidomics of small biological organisms and cells using mass spectrometry. Anal. Chem. 2018, 90, 6936–6944. [Google Scholar] [CrossRef] [PubMed]
- Souza-Silva, E.A.; Pawliszyn, J. Optimization of fiber coating structure enables direct immersion solid-phase microextraction and high-throughput determination of complex samples. Anal. Chem. 2012, 84, 6933–6938. [Google Scholar] [CrossRef] [PubMed]
- Souza-Silva, E.A.; Pawliszyn, J. Direct immersion solid-phase microextraction with matrix-compatible fiber coating for multiresidue pesticide analysis of grapes by gas chromatography-time-of-flight mass spectrometry (DI-SPME-GC-ToFMS). J. Agric. Food Chem. 2015, 63, 4464–4477. [Google Scholar] [CrossRef]
- Souza-Silva, E.A.; Lopez-Avila, V.; Pawliszyn, J. Fast and robust direct immersion solid-phase microextraction coupled with gas chromatography-time-of-flight mass spectrometry method employing a matrix compatible fiber for determination of triazole fungicides in fruits. J. Chromatogr. A 2013, 1313, 139–146. [Google Scholar] [CrossRef]
- Naccarato, A.; Pawliszyn, J. Matrix compatible solid-phase microextraction coating, a greener approach to sample preparation in vegetable matrices. Food Chem. 2016, 206, 67–73. [Google Scholar] [CrossRef]
- De Grazia, S.; Gionfriddo, E.; Pawliszyn, J. A new and efficient solid-phase microextraction approach for analysis of high fat content food samples using a matrix-compatible coating. Talanta 2017, 167, 754–760. [Google Scholar] [CrossRef] [Green Version]
- Stenerson, K.; Young, T.; Shirey, R.; Chen, Y.; Sidisky, L. Overcoated PDMS-DVB fiber to the extraction of pesticides from spaghetti sauce: Method evaluation and comparison to QuEChERS. LC-GC N. Am. 2016, 34, 500–509. [Google Scholar]
- Stenerson, K.; Young, T. Analysis of pesticides in baby food by solid-phase microextraction (SPME) using a new overcoated fiber. Rep. Appl. Newsl. 2016, 34, 15–19. [Google Scholar]
- Zhang, L.; Gionfriddo, E.; Acquaro, V.; Pawliszyn, J. Direct immersion solid-phase microextraction analysis of multi-class contaminants in edible seaweeds by gas chromatography-mass spectrometry. Anal. Chim. Acta 2018, 1031, 83–97. [Google Scholar] [CrossRef] [Green Version]
- Gionfriddo, E.; Passarini, A.; Pawliszyn, J. A facile and fully automated on-fiber derivatization protocol for direct analysis of short-chain aliphatic amines using a matrix compatible solid-phase microextraction coating. J. Chromatogr. A 2016, 1457, 22–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirabelli, M.F.; Gionfriddo, E.; Pawliszyn, J.; Zenobi, R. A quantitative approach for pesticide analysis in grape juice by direct interfacing of a matrix compatible SPME phase to dielectric barrier discharge ionization-mass spectrometry. Analyst 2018, 143, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Godage, N.H.; Gionfriddo, E. A critical outlook on recent developments and applications of matrix compatible coatings for solid-phase microextraction. TrAC Trends Anal. Chem. 2019, 111, 220–228. [Google Scholar] [CrossRef]
- Ouyang, G.; Vuckovic, D.; Pawliszyn, J. Nondestructive sampling of living systems using in vivo solid-phase microextraction. Chem. Rev. 2011, 111, 2784–2814. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, G.; Oakes, K.D.; Bragg, L.; Wang, S.; Liu, H.; Cui, S.; Servos, M.R.; Dixon, D.G.; Pawliszyn, J. Sampling-rate calibration for rapid and nonlethal monitoring of organic contaminants in fish muscle by solid-phase microextraction. Environ. Sci. Technol. 2011, 45, 7792–7798. [Google Scholar] [CrossRef]
- Nicklas, T.A.; O’Neil, C.E.; Fulgoni, V.L. Consumption of various forms of apples is associated with a better nutrient intake and improved nutrient adequacy in diets of children: National Health and Nutrition Examination Survey 2003-2010. Food Nutr. Res. 2015, 59, 25948. [Google Scholar] [CrossRef] [Green Version]
- Giaretta, A.G.; Schulz, M.; Silveira, T.T.; de Oliveira, M.V.; Patricio, M.J.; Gonzaga, L.V.; Fett, R.; da Silva, E.L.; Wazlawik, E. Apple intake improves antioxidant parameters in hemodialysis patients without affecting serum potassium levels. Nutr. Res. 2019, 64, 56–63. [Google Scholar] [CrossRef]
- Bagherian, A.; Ghorbani, M.; Mirzababaei, S.N. Nickel ion removal using nanoporous poly(styrene-co divinyl benzene) copolymer. Korean J. Chem. Eng. 2015, 32, 2153–2160. [Google Scholar] [CrossRef]
- Garcia-Diego, C.; Cuellar, J. Application of cluster analysis and optimization to determine the synthesis conditions of macroreticular poly(styrene-co-divinylbenzene) microparticles with enhanced structural and adsorption properties. Chem. Eng. J. 2008, 139, 198–207. [Google Scholar] [CrossRef]
- Mukhtar, M.; Sherwani, A.; Wani, A.A.; Ahmed, S.B.; Sofi, J.A.; Bano, P. Degradation of chlorpyrifos residues in apple under temperate conditions of Kashmir Valley. Environ. Monit. Assess. 2015, 187, 482. [Google Scholar] [CrossRef]
- Behkami, S. Statistical and chemometric view of the variation in the concentration of selected organophosphates in peeled unwashed and unpeeled washed fruits and vegetables. Food Chem. 2019, 300, 125220. [Google Scholar] [CrossRef] [PubMed]
- Rasmusssen, R.R.; Poulsen, M.E.; Hansen, H.C.B. Distribution of multiple pesticide residues in apple segments after home processing. Food Addit. Contam. Part A-Chem. 2003, 20, 1044–1063. [Google Scholar] [CrossRef] [PubMed]
- Cherta, L.; Beltran, J.; Pitarch, E.; Hernandez, F. Comparison of simple and rapid extraction procedures for the determination of pesticide residues in fruit juices by fast gas chromatography–mass spectrometry. Food Anal. Meth. 2013, 6, 1671–1684. [Google Scholar] [CrossRef] [Green Version]
- Reddy, A.V.B.; Yusop, Z.; Jaafar, J.; Bin Aris, A.; Majid, Z.A.; Umar, K.; Talib, J. Simultaneous determination of three organophosphorus pesticides in different food commodities by gas chromatography with mass spectrometry. J. Sep. Sci. 2016, 39, 2276–2283. [Google Scholar] [CrossRef]
- Farajzadeh, M.A.; Mohebbi, A.; Mogaddam, M.R.A.; Davaran, M.; Norouzi, M. Development of salt-induced homogenous liquid–liquid microextraction based on iso-propanol/sodium sulfate system for extraction of some pesticides in fruit juices. Food Anal. Meth. 2018, 11, 2497–2507. [Google Scholar] [CrossRef]
- Mohammadi, V.; Saraji, M.; Jafari, M.T. Direct molecular imprinting technique to synthesize coated electrospun nanofibers for selective solid-phase microextraction of chlorpyrifos. Microchim. Acta 2019, 186, 524. [Google Scholar] [CrossRef]
- Gonzalez-Rodriguez, M.J.; Liebanas, F.J.A.; Frenich, A.G.; Vidal, J.L.M.; Lopez, F.J.S. Determination of pesticides and some metabolites in different kinds of milk by solid-phase microextraction and low-pressure gas chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2005, 382, 164–172. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |









| Analyte | Linear Range (ng/g) | R2 | LOD a (ng/g) | LOQ b (ng/g) | RSD c (%) | Spiking Level (ng/g) | Recovery (%) |
|---|---|---|---|---|---|---|---|
| Diazinon | 1.25–25 | 0.9996 | 0.02 | 0.07 | 8.11 | 2.5 | 89.90 ± 4.89 |
| 12.5 | 86.31 ± 9.21 | ||||||
| 25 | 87.56 ± 11.96 | ||||||
| Chlorpyrifos | 1.25–250 | 0.9991 | 0.10 | 0.33 | 4.08 | 12.5 | 74.82 ± 6.64 |
| 25 | 90.02 ± 8.91 | ||||||
| 125 | 98.50 ± 8.84 |
| Analyte | Sampling Time (min) | Concentration (µg/g) | RSD% |
|---|---|---|---|
| Diazinon | 3 10 20 | 1.61 ± 0.04 1.70 ± 0.11 1.64 ± 0.06 | 4.92 |
| Chlorpyrifos | 3 10 20 | 2.10 ± 0.01 2.10 ± 0.01 2.10 ± 0.01 | 0.30 |
| Analyte | t (min) | Concentration (µg/g) | RSD% | |
|---|---|---|---|---|
| In situ SPME/GC | LLE/GC | |||
| Diazinon | 15 | 1.62 ± 0.28 a | 2.62 ± 0.31 | 29.21 |
| 18 | 2.02 ± 0.48 b | 2.07 ± 0.12 | 1.7 | |
| 20 | 1.26 ± 0.16 c | 1.24 ± 0.10 | 0.95 | |
| 22 | 1.19 ± 0.16 c | 1.36 ± 0.03 | 9.25 | |
| 25 | 1.12 ± 0.16 c | 1.54 ± 0.01 | 22.24 | |
| 30 | 1.02 ± 0.39 c | 1.73 ± 0.12 | 38.54 | |
| 40 | 0.90 ± 0.30 c | 1.64 ± 0.03 | 39.65 | |
| Chlorpyrifos | 15 | 4.20 ± 0.31 a | 5.09 ± 0.56 | 12.38 |
| 18 | 5.02 ± 0.25 b | 5.06 ± 0.39 | 0.59 | |
| 20 | 3.62 ± 0.12 c | 3.25 ± 0.02 | 8.87 | |
| 22 | 3.42 ± 0.09 c | 3.58 ± 0.09 | 3.39 | |
| 25 | 3.12 ± 0.11 c | 3.82 ± 0.10 | 14.4 | |
| 30 | 2.51 ± 0.14 d | 3.92 ± 0.22 | 30.26 | |
| 40 | 1.95 ± 0.19 e | 4.05 ± 0.11 | 40.75 | |
| Analyte | LOD (ng/g) | LOQ (ng/g) | RSD% |
|---|---|---|---|
| Diazinon | 0.18 | 0.60 | 2.28 |
| Chlorpyrifos | 0.20 | 0.67 | 0.28 |
| Analyte | Soaking Concentration (µg/mL) | Concentration (µg/g) | RSD% | |
|---|---|---|---|---|
| In Situ SPME/GC | LLE/GC | |||
| Diazinon | 20 | 1.32 ± 0.02 | 1.13 ± 0.01 | 7.9 |
| 40 | 1.58 ± 0.12 | 1.75 ± 0.01 | 7.75 | |
| 60 | 1.64 ± 0.02 | 1.84 ± 0.09 | 6.04 | |
| Chlorpyrifos | 20 | 2.73 ± 0.01 | 2.22 ± 0.02 | 9.79 |
| 40 | 2.80 ± 0.02 | 2.88 ± 0.32 | 6.22 | |
| 60 | 2.62 ± 0.02 | 2.85 ± 0.09 | 4.91 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.-F.; Zhao, L.-L.; Huang, M.-Q.; Li, X.-J.; Pan, S.-Y. In Situ Real-Time Tracing of Organophosphorus Pesticides in Apples by Solid-Phase Microextraction with Developed Sampling-Rate Calibration. Molecules 2019, 24, 4444. https://doi.org/10.3390/molecules24244444
Zhang X-F, Zhao L-L, Huang M-Q, Li X-J, Pan S-Y. In Situ Real-Time Tracing of Organophosphorus Pesticides in Apples by Solid-Phase Microextraction with Developed Sampling-Rate Calibration. Molecules. 2019; 24(24):4444. https://doi.org/10.3390/molecules24244444
Chicago/Turabian StyleZhang, Xiao-Fan, Li-Li Zhao, Ming-Quan Huang, Xiu-Juan Li, and Si-Yi Pan. 2019. "In Situ Real-Time Tracing of Organophosphorus Pesticides in Apples by Solid-Phase Microextraction with Developed Sampling-Rate Calibration" Molecules 24, no. 24: 4444. https://doi.org/10.3390/molecules24244444