Possible Mechanisms of Biological Effects Observed in Living Systems during 2H/1H Isotope Fractionation and Deuterium Interactions with Other Biogenic Isotopes
Abstract
:1. Introduction
2. Molecular Effects of 2H/1H Exchange
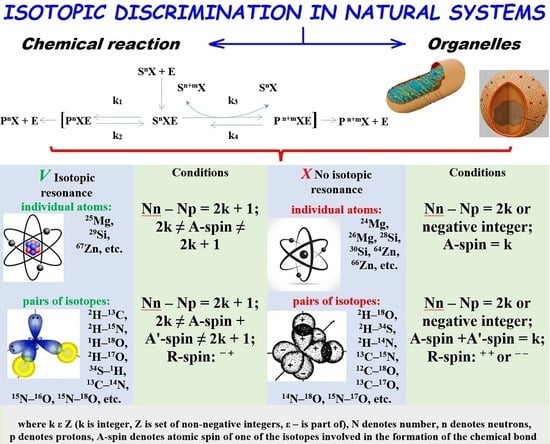
3. Isotope Exchange Effects Observed at the Organelles
4. In Vivo 2H/1H Exchange Effects
5. 2H/1H Exchange Effects and Other Heavy Nonradioactive Isotopic Fractionations in Living and Natural Systems via Neutron Effect Realization (Hypothesis)
6. Possibility of Using the Isotopic Therapy in 2H-Modified Drinking and Diet for Some Diseases
- −
- −
- Parkinson’s disease was partially cured by the administration of D-PUFAs, which protected against nigrostriatal damage (in the substantia nigra) from oxidative injury in mice with a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model [119];
- −
- Mitochondrial disorders and oxidative stress were corrected by both deuterium-depleted water [16,55,56,65,69,120,121] and D-PUFAs, such as 11,11-D(2)-linoleic and 11,11,14,14-D(4)-α-linolenic acids and others, which preserved mitochondrial bioenergetics function under toxic effects of t-butylhydroperoxide, ethacrynic acid, or ferrous iron [122], prevented coenzyme Q (ubiquinone or CoQ)-less coq mutants and some toxic effects of lipid autoxidation products [123,124,125], and also decelerated the aging process by deuterated trilinolenin (D-TG (54:9)), resulting in extended lifespan of worms under normal and oxidative stress conditions [126];
- −
- −
- −
- Huntington’s disease was managed by D-PUFA diet in Q140 knock-in mice, which were able to demonstrate improved performance in novel object recognition tests after 5 months of consumption of deuterium-enriched diet [129];
- −
- Cancer adjuvant therapy showed efficacy through the administration of 1H2O [22,70,130,131], and previously-drug-processed gliomas was treated by combining deuterium-depleted drinking and a lower deuterated diet, which led to intracellular deuterium disequilibrium and formation of the 2H/1H gradient [62,132];
- −
- Inflammatory diseases were fine-tuned to potentially various inflammation stages using deuterated isotopologues of eicosanoids (for example, arachidonic acids with 2H at the bis-allylic C7, C10, and C13 positions [133]);
- −
- −
- Cardiovascular diseases were treated by improving cholesterol handling and reducing atherosclerosis development using D-PUFAs, which decreased lipid peroxidation, body weight gain, and plasma and hepatic cholesterol contents [136], as well as by using D-PUFAs to reduce the tocopherol-mediated peroxidation of lipids in human low-density lipoproteins [137].
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schmidt, H.L.; Robins, R.J.; Werner, R.A. Multi-factorial in vivo stable isotope fractionation: Causes, correlations, consequences and applications. Isot. Environ. Health Stud. 2015, 51, 155–199. [Google Scholar] [CrossRef] [PubMed]
- Basov, A.A.; Fedulova, L.V.; Baryshev, M.G.; Dzhimak, S.S. Deuterium-depleted water influence on the isotope 2H/1H regulation in body and individual adaptation. Nutrients 2019, 11, 1903. [Google Scholar] [CrossRef] [PubMed]
- Galimov, E.M. On an erroneous “experience” of isotope fractionation in enzymatic reaction. Russ. J. Phys. Chem. A 2007, 81, 831–835. [Google Scholar] [CrossRef]
- Brenna, J.T. Natural intramolecular isotope measurements in physiology: Elements of the case for an effort toward high-precision position-specific isotope analysis. Rapid Commun. Mass Spectrom. 2001, 15, 1252–1262. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.M. Isotopic order, biogeochemical processes, and earth history. Geochim. Cosmochim. Acta 2004, 68, 1691–1700. [Google Scholar] [CrossRef]
- Walter, W.D.; Kurle, C.M.; Hopkins, J.B. Applications of stable isotope analysis in mammalian ecology. Isot. Environ. Health Stud. 2014, 50, 287–290. [Google Scholar] [CrossRef]
- Kosenkov, A.V.; Gulyaev, M.V.; Anisimov, N.V.; Lobyshev, V.I.; Pirogov, Y.A. Investigation of the distribution of heavy nuclei in laboratory animals using multinuclear magnetic resonance imaging. Phys. Wave Phenom. 2015, 23, 311–315. [Google Scholar] [CrossRef]
- Pedersen, L.G.; Bartolotti, L.; Li, L. Deuterium and its role in the machinery of evolution. J. Theor. Biol. 2006, 238, 914–918. [Google Scholar] [CrossRef]
- Podlesak, D.W.; Torregrossa, A.-M.; Ehleringer, J.R.; Dearing, M.D.; Passey, B.H.; Cerling, T.E. Turnover of oxygen and hydrogen isotopes in the body water, CO2, hair, and enamel of a small mammal. Geochim. Cosmochim. Acta 2008, 72, 19–35. [Google Scholar] [CrossRef]
- Podlesak, D.W.; Bowen, G.J.; O’Grady, S.; Cerling, T.E.; Ehleringer, J.R. δ2H and δ18O of human body water: A GIS model to distinguish residents from non-residents in the contiguous USA. Isot. Environ. Health Stud. 2012, 48, 259–279. [Google Scholar] [CrossRef]
- Kelly, S.; Heaton, K.; Hoogewerff, J. Tracing the geographical origin of food: The application of multi-element and multi-isotope analysis. Trends Food Sci. Technol. 2005, 16, 555–567. [Google Scholar] [CrossRef]
- Hobson, K.A.; Bond, A.L. Extending an indicator: Year-round information on seabird trophic ecology from multiple-tissue stable-isotope analyses. Mar. Ecol. Prog. Ser. 2012, 461, 233–243. [Google Scholar] [CrossRef]
- Galimov, E.M. Life is a product of molecular ordering “machine”. Geochem. Int. 2014, 52, 1190–1196. [Google Scholar] [CrossRef]
- Ivlev, A.A.; Knyazev, Y.A.; Logachev, M.F. Short-period variations in carbon isotope composition of CO2 of expired air under different physiological conditions in man. Biofizika 1996, 41, 508–516. [Google Scholar] [PubMed]
- Frolov, V.Y.; Baryshev, M.G.; Bolotin, S.N.; Dzhimak, S.S. Method of Producing Biologically Active Drinking Water with Low Deuterium Content. Patent RF 2438765, 10 January 2012. [Google Scholar]
- Rasooli, A.; Fatemi, F.; Akbarzadeh, K.; Dini, S.; Bahremand, S. Synergistic protective activity of deuterium depleted water (DDW) and saturejarechingeri essential oil on hepatic oxidative injuries induced by acetaminophen in rats. J. Essent. Oil Bear. Plants 2016, 19, 1086–1101. [Google Scholar] [CrossRef]
- Lisitsy, A.B.; Bogatyrev, A.N.; Dydykin, A.S.; Derevitskaya, O.K.; Soldatova, N.E.; Fedulova, L.V. Influence of meat semiprepared foods produced with an addition of water having a reduced deuterium content on the indicators of the laboratory animals with the model of alloxan diabetes. Vopr. Pitan. 2017, 86, 64–71. [Google Scholar]
- Chesson, L.A.; Valenzuela, L.O.; O’Grady, S.P.; Cerling, T.E.; Ehleringer, J.R. Hydrogen and oxygen stable isotope ratios of milk in the United States. J. Agric. Food Chem. 2010, 58, 2358–2363. [Google Scholar] [CrossRef]
- Bowen, G.J.; Winter, D.A.; Spero, H.J.; Zierenberg, R.A.; Reeder, M.D.; Cerling, T.E.; Ehleringer, J.R. Stable hydrogen and oxygen isotope ratios of bottled waters of the world. Rapid Commun. Mass Spectrom. 2005, 19, 3442–3450. [Google Scholar] [CrossRef]
- Mladin, C.; Ciobica, A.; Lefter, R.; Popescu, A.; Bild, W. Deuterium depletion induces anxiolytic-like effects in rats. Arch. Biol. Sci. 2014, 66, 947–953. [Google Scholar] [CrossRef]
- Stefanescu, I.; Manolescu, N.; Titescu, G.; Balint, E.; Tamaian, R.; Feurdean, V. Assessment of deuterium depletion in plymouth rock chickens’ bodies. Environ. Eng. Manag. J. 2010, 9, 1477–1480. [Google Scholar]
- Gyöngyi, Z.; Budán, F.; Szabó, I.; Ember, I.; Kiss, I.; Krempels, K.; Somlyai, I.; Somlyai, G. Deuterium depleted water effects on survival of lung cancer patients and expression of Kras, Bcl2, and Myc genes in mouse lung. Nutr. Cancer 2013, 65, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Dzhimak, S.S.; Basov, A.A.; Elkina, A.A.; Fedulova, L.V.; Kotenkova, E.A.; Vasilevskaya, E.R.; Lyasota, O.M.; Baryshev, M.G. Influence of deuterium-depleted water on hepatorenal toxicity. Jundishapur. J. Nat. Pharm. Prod. 2018, 13, e69557. [Google Scholar] [CrossRef]
- Storm-Suke, A.; Wassenaar, L.I.; Nol, E.; Norris, D.R. The influence of metabolic rate on the contribution of stable-hydrogen and oxygen isotopes in drinking water to quail blood plasma and feathers. Funct. Ecol. 2012, 26, 1111–1119. [Google Scholar] [CrossRef]
- Lobyshev, V.I.; Kalinichenko, L.P. Isotopic Effects of D2O in Biological Systems; Nauka: Moscow, Russia, 1978; p. 215. [Google Scholar]
- Buchachenko, A.L. Photooxidation of water by molecular oxygen: Isotope exchange. Theor. Exp. Chem. 2011, 46, 359–362. [Google Scholar] [CrossRef]
- Buchachenko, A.L.; Kuznetsov, D.A.; Breslavskaya, N.N.; Shchegoleva, L.N.; Arkhangelsky, S.E. Calcium induced ATP synthesis: Isotope effect, magnetic parameters and mechanism. Chem. Phys. Lett 2011, 505, 130–134. [Google Scholar] [CrossRef]
- Buchachenko, A.L.; Kuznetsov, D.A.; Breslavskaya, N.N. Chemistry of enzymatic ATP synthesis: An insight through the isotope window. Chem. Rev. 2012, 112, 2042–2058. [Google Scholar] [CrossRef]
- Buchachenko, A.L.; Kuznetsov, D.A. Efficiency of ATP synthase as a molecular. Biophysics 2008, 53, 219–222. [Google Scholar] [CrossRef]
- Buchachenko, A.L.; Orlov, A.P.; Kuznetsov, D.A.; Breslavskaya, N.N. Magnetic control of the DNA synthesis. Chem. Phys. Lett 2013, 586, 138–142. [Google Scholar] [CrossRef]
- Buchachenko, A.L. Magnetic Isotope Effect in Chemistry and Biochemistry; Nova Science Publ.: New York, NY, USA, 2009; p. 105. [Google Scholar]
- Koltover, V.K.; Labyntseva, R.D.; Kosterin, S.O.; Karandashev, V.K. Magnetic isotope of magnesium accelerates ATP hydrolysis catalyzed by myosin. Biophysics 2016, 61, 200–206. [Google Scholar] [CrossRef]
- Koltover, V.K.; Avdeeva, L.V.; Kudryashova, E.A.; Shevchenko, U.G.; Royba, E.A.; Berdinsky, V.L. Magnetic-isotope effect of magnesium in the living cell. Dokl. Biochem. Biophys. 2012, 442, 12–14. [Google Scholar] [CrossRef]
- Xie, X.; Zubarev, R.A. Effects of low-level deuterium enrichment on bacterial growth. PLoS ONE 2014, 9, e102071. [Google Scholar] [CrossRef] [PubMed]
- Nikitin, D.I.; Oranskaya, M.N.; Lobyshev, V.I. Specificity of bacterial response to variations of the isotopic composition of water. Biophysics 2003, 48, 636–640. [Google Scholar]
- Stefanescu, I.; Manolescu, N.; Titescu, G.; Comisel, V.; Tamaian, R.; Feurdean, V. Studies concerning DDW use for deuterium depletion in the organism of laboratory animals. Environ. Eng. Manag. J. 2010, 9, 1473–1476. [Google Scholar] [CrossRef]
- Rodin, S.; Rebellato, P.; Lundin, A.; Zubarev, R.A. Isotopic resonance at 370 ppm deuterium negatively affects kinetics of luciferin oxidation by luciferase. Sci. Rep. 2018, 8, 16249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ek, C.; Garbaras, A.; Yu, Z.; Oskarsson, H.; Wiklund, A.-K.E.; Kumblad, L.; Gorokhova, E. Increase in stable isotope ratios driven by metabolic alterations in amphipods exposed to the beta-blocker propranolol. PLoS ONE 2019, 14, e0211304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchachenko, A.L.; Pliss, E.M. Hydrogen isotope effects and atom tunneling. Russ. Chem. Rev. 2016, 85, 557–564. [Google Scholar] [CrossRef]
- Shchepinov, M.S. Reactive oxygen species, isotope effect, essential nutrients, and enhanced longevity. Rejuvenation Res. 2007, 10, 47–59. [Google Scholar] [CrossRef]
- Li, X.; Snyder, M.P. Yeast longevity promoted by reversing aging-associated decline in heavy isotope content. Npj Aging Mech. Dis. 2016, 2, 16004. [Google Scholar] [CrossRef]
- Heazlewood, B.R.; Maccarone, A.T.; Andrews, D.U.; Osborn, D.L.; Harding, L.B.; Klippenstein, S.J.; Jordan, M.J.; Kable, S.H. Near-threshold H/D exchange in CD3CHO photodissociation. Nat. Chem. 2011, 3, 443–448. [Google Scholar] [CrossRef]
- Andrianova, Z.S.; Buchachenko, A.L.; Breslavskaya, N.N.; Pliss, E.M. Bond energies in polyunsaturated acids and kinetics of co-oxidation of protiated and deuterated acids. Russ. J. Phys. Chem. A 2016, 90, 1936–1941. [Google Scholar] [CrossRef]
- Martin, G.J.; Zhang, B.L.; Naulet, N.; Martin, M.L. Deuterium transfer in the bioconversion of glucose to ethanol studied by specific isotope labeling at the natural abundance level. J. Am. Chem. Soc. 1986, 108, 5116–5122. [Google Scholar] [CrossRef]
- Zhang, X.; Gillespie, A.L.; Sessions, A.L. Large D/H variations in bacterial lipids reflect central metabolic pathways. Proc. Natl. Acad. Sci. USA 2009, 106, 12580–12586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, C.R.; Bowen, B.P.; Pan, C.; Northen, T.R.; Banfield, J.F. Stable-isotope probing reveals that hydrogen isotope fractionation in proteins and lipids in a microbial community are different and species-specific. ACS Chem. Biol. 2013, 8, 1755–1763. [Google Scholar] [CrossRef] [PubMed]
- Bila, W.C.; Mariano, R.M.S.; Silva, V.R.; dos Santos, M.E.S.M.; Lamounier, J.A.; Ferriolli, E.; Galdino, A.S. Applications of deuterium oxide in human health. Isot. Environ. Health Stud. 2017, 53, 327–343. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.R.; Johansen, E.; Holman, T.R. Large competitive kinetic isotope effects in human 15-lipoxygenase catalysis measured by a novel HPLC method. J. Am. Chem. Soc. 1999, 121, 1395–1396. [Google Scholar] [CrossRef]
- Uspenskaya, E.V.; Anfimova, E.V.; Syroeshkin, A.V.; Pleteneva, T.V. Kinetics of active pharmaceutical ingredient solubility in water with different hydrogen isotopic content. Indian J. Pharm. Sci. 2018, 80, 318–324. [Google Scholar] [CrossRef]
- Vasilevskaya, E.R.; Akhremko, A.G. Proteomic study of pig’s spleen. Potravin. Slovak J. Food Sci. 2019, 13, 314–317. [Google Scholar] [CrossRef]
- Fedulova, L.V.; Vasilevskaya, E.R.; Kotenkova, E.A.; Elkina, A.A.; Baryshev, M.G.; Lisitsyn, A.B. Influence of different polypeptides fractions derived from Sus Scrofa immune organs on the rats immunological reactivity. J. Pharm. Nutr. Sci. 2017, 7, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Fedulova, L.V.; Basov, A.A.; Vasilevskaya, E.R.; Dzhimak, S.S. Gender difference response of male and female immunodeficiency rats treated with tissue-specific biomolecules. Curr. Pharm. Biotechnol. 2019, 20, 245–253. [Google Scholar] [CrossRef]
- Fedulova, L.; Elkina, A.; Vasilevskaya, E.; Barysheva, E. Identification of tissue-specific proteins of immunocompetent organs of Sus scrofa isolated in deuterium depleted medium. Med. Sci. 2018, 22, 509–513. [Google Scholar]
- Darad, R.; Aiyar, A.S. Effects of heavy water on mitochondrial respiration and oxidative phosphorylation. J. Biosci. 1982, 4, 159–166. [Google Scholar] [CrossRef]
- Kolesova, O.E.; Pomytkin, I.A. Relationship between natural concentration of heavy water isotopologs and rate of H2O2 generation by mitochondria. Bull. Exp. Biol. Med. 2006, 142, 570–572. [Google Scholar]
- Dzhimak, S.S.; Basov, A.A.; Volchenko, N.N.; Samkov, A.A.; Fedulova, L.V.; Baryshev, M.G. Changes in the functional activity of mitochondria isolated from the liver of rat that passed the preadaptation to ultra-low deuterium concentration. Dokl. Biochem. Biophys. 2017, 476, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Olgun, A. Biological effects of deuteronation: ATP synthase as an example. Theor. Biol. Med. Model. 2007, 4, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wanders, R.J.A.; Waterham, H.R.; Ferdinandusse, S. Metabolic interplay between peroxisomes and other subcellular organelles including mitochondria and the endoplasmic reticulum. Front. Cell Dev. Biol. 2015, 3, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotkina, I.T.; Titov, V.N.; Parkhimovich, R.M. The different notions about beta-oxidation of fatty acids in peroxisomes, peroxisomes and ketonic bodies. The diabetic, acidotic coma as an acute deficiency of acetyl-CoA and ATP. Klin. Lab. Diagn 2014, 3, 14–23. [Google Scholar]
- Pollak, N.; Dolle, C.; Ziegler, M. The power to reduce: Pyridine nucleotides—small molecules with a multitude of functions. BioChem. J. 2007, 402, 205–218. [Google Scholar] [CrossRef]
- Lewis, C.A.; Parker, S.J.; Fiske, B.P.; McCloskey, D.; Gui, D.Y.; Green, C.R.; Vokes, N.I.; Feist, A.M.; Heiden, M.G.V.; Metallo, C.M. Tracing compartmentalized NADPH metabolism in the cytosol and mitochondria of mammalian cells. Mol. Cell 2014, 55, 253–263. [Google Scholar] [CrossRef] [Green Version]
- Boros, L.G.; D’Agostino, D.P.; Katz, H.E.; Roth, J.P.; Meuillet, E.J.; Somlyai, G. Submolecular regulation of cell transformation by deuterium depleting water exchange reactions in the tricarboxylic acid substrate cycle. Med. Hypotheses 2016, 87, 69–74. [Google Scholar] [CrossRef]
- Sormendi, S.; Wielockx, B. Hypoxia pathway proteins as central mediators of metabolism in the tumor cells and their microenvironment. Front. Immunol. 2018, 9, 40. [Google Scholar] [CrossRef]
- Strekalova, T.; Evans, M.; Chernopiatko, A.; Couch, Y.; Costa-Nunes, J.; Cespuglio, R.; Chesson, L.; Vignisse, J.; Steinbusch, H.W.; Anthony, D.C.; et al. Deuterium content of water increases depression susceptibility: The potential role of a serotonin-related mechanism. Behav. Brain Res. 2015, 277, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Avila, D.S.; Somlyai, G.; Somlyai, I.; Aschner, M. Anti-aging effects of deuterium depletion on Mn-induced toxicity in a C. elegans model. Toxicol. Lett. 2012, 211, 319–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Letuta, U.G.; Berdinskiy, V.L. Magnetosensitivity of bacteria E. coli: Magnetic isotope and magnetic field effects. Bioelectromagnetics 2017, 38, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Cong, F.; Zhang, Y.; Sheng, H.; Ao, Z.; Zhang, S.; Wang, J. Deuterium-depleted water inhibits human lung carcinoma cell growth by apoptosis. Exp. Ther. Med. 2010, 1, 277–283. [Google Scholar] [CrossRef] [Green Version]
- Kozin, S.V.; Kravtsov, A.A.; Elkina, A.A.; Zlishcheva, E.I.; Barysheva, E.V.; Shurygina, L.V.; Moiseev, A.V.; Baryshev, M.G. Isotope exchange of deuterium for protium in rat brain tissues changes brain tolerance to hypoxia. Biophysics 2019, 64, 272–278. [Google Scholar] [CrossRef]
- Rasooli, A.; Fatemi, F.; Hajihosseini, R.; Vaziri, A.; Akbarzadeh, K.; Mohammadi Malayeri, M.R.; Dini, S.; Foroutanrad, M. Synergistic effects of deuterium depleted water and Mentha longifolia L. essential oils on sepsis-induced liver injuries through regulation of cyclooxygenase-2. Pharm. Biol. 2019, 57, 125–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Gaetani, M.; Chernobrovkin, A.; Zubarev, R.A. Anticancer effect of deuterium depleted water—Redox disbalance leads to oxidative stress. Mol. Cell Proteom. 2019. [Google Scholar] [CrossRef] [Green Version]
- Shchepinov, M.S. Do “heavy” eaters live longer? BioEssays 2007, 29, 1247–1256. [Google Scholar] [CrossRef]
- Dzhimak, S.S.; Basov, A.A.; Baryshev, M.G. Content of deuterium in biological fluids and organs: Influence of deuterium depleted water on D/H gradient and the process of adaptation. Dokl. Biochem. Biophys. 2015, 465, 370–373. [Google Scholar] [CrossRef]
- O’Grady, S.P.; Wende, A.R.; Remien, C.H.; Valenzuela, L.O.; Enright, L.E.; Chesson, L.A.; Dale Abel, E.; Cerling, T.E.; Ehleringer, J.R. Aberrant water homeostasis detected by stable isotope analysis. PLoS ONE 2010, 5, e11699. [Google Scholar] [CrossRef]
- Somlyai, G.; Jancsó, G.; Jákli, G.; Vass, K.; Barna, B.; Lakics, V.; Gaál, T. Naturally occurring deuterium is essential for the normal growth rate of cells. FEBS Lett. 1993, 317, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Kravtsov, A.A.; Kozin, S.V.; Vasilevskaya, E.R.; Elkina, A.A.; Fedulova, L.V.; Popov, K.A.; Malyshko, V.V.; Moiseev, A.V.; Shashkov, D.I.; Baryshev, M.G. Effect of drinking ration with reduced deuterium content on brain tissue prooxidant-antioxidant balance in rats with acute hypoxia model. J. Pharm. Nutr. Sci. 2018, 8, 42–51. [Google Scholar] [CrossRef]
- Samkov, A.A.; Dzhimak, S.S.; Barishev, M.G.; Volchenko, N.N.; Khudokormov, A.A.; Samkova, S.M.; Karaseva, E.V. The effect of water isotopic composition on Rhodococcus erythropolis biomass production. Biophysics 2015, 60, 107–112. [Google Scholar] [CrossRef]
- Letuta, U.G.; Letuta, S.N.; Berdinskiy, V.L. The influence of low magnetic fields and magnesium isotopes on E. coli bacteria. Biophysics 2017, 62, 935–941. [Google Scholar] [CrossRef]
- Bild, W.; Năstasă, V.; Haulică, I. In vivo and in vitro research on the biological effects of deuterium-depleted water: 1. Influence of deuterium-depleted water on cultured cell growth. Rom. J. Physiol. 2004, 41, 53–67. [Google Scholar]
- Basov, A.A.; Elkina, A.A.; Samkov, A.A.; Volchenko, N.N.; Moiseev, A.V.; Fedulova, L.V.; Baryshev, M.G.; Dzhimak, S.S. Influence of deuterium depleted water on the isotope D/H composition of liver tissue and morphological development of rats at different periods of ontogenesis. Iran. Biomed. J. 2019, 23, 129–141. [Google Scholar] [CrossRef]
- Petrus-Vancea, A. Cell ultrastructure and chlorophyll pigments in hyperhydric and non-hyperhydric Beta vulgaris var. Conditiva plantlets, treated with deuterium depleted water. Plant Cell Tissue Organ. Cult. 2018, 135, 13–21. [Google Scholar] [CrossRef]
- Kosenkov, A.V.; Gulyaev, M.V.; Lobyshev, V.I.; Yusubalieva, G.M.; Baklaushev, V.P. The reversible effect of deuteration on tissue fluid and biopolymers in normal and tumor tissues of mice. Biophysics 2018, 63, 820–824. [Google Scholar] [CrossRef]
- Yavari, K.; Kooshesh, L. Deuterium depleted water inhibits the proliferation of human MCF7 breast cancer cell lines by inducing cell cycle arrest. Nutr. Cancer 2019. [Google Scholar] [CrossRef]
- Lobyshev, V.I. Biphasic response of biological objects on variation of low deuterium concentration in water. Int. J. High Dilution Res. 2018, 17, 12–13. [Google Scholar]
- Xie, X.; Zubarev, R.A. Isotopic resonance hypothesis: Experimental verification by Escherichia coli growth measurements. Sci. Rep. 2015, 5, 9215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crotty, F.V.; Blackshaw, R.P.; Murray, P.J. Differential growth of the fungus Absidia cylindrospora on 13C/15N-labelled media. Rapid Comm. Mass Spectrom. 2011, 25, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Dennis, C.A.; MacNeil, M.A.; Rosati, J.Y.; Pitcher, T.E.; Fisk, A.T. Diet discrimination factors are inversely related to δ15N and δ13C values of food for fish under controlled conditions. Rapid Commun. Mass. Spectrom. 2010, 24, 3515–3520. [Google Scholar] [CrossRef] [PubMed]
- Andriukonis, E.; Gorokhova, E. Kinetic 15N-isotope effects on algal growth. Sci. Rep. 2017, 7, 44181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Letuta, U.G.; Tikhonova, T.A. Magnetic fields and magnetic isotope 25Mg effects on biofilms formation by bacteria E. coli. Dokl. Biochem. Biophys. 2019, 484, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Waki, M.; Miyamoto, K. Isotope Enrichment Method. U.S. Patent 7,943,107, 17 May 2011. [Google Scholar]
- Letuta, U.G.; Shailina, D.M. Magnetosensitivity of E. coli bacteria in the presence of zinc isotopes. Dokl. Biochem. Biophys. 2018, 479, 111–113. [Google Scholar] [CrossRef]
- Letuta, U.G.; Berdinskiy, V.L. Effects of the magnetic field and zinc isotopes on the colony forming ability and elemental composition of E. coli bacterial cells. Russ. Chem. Bull. 2018, 67, 1732–1737. [Google Scholar] [CrossRef]
- Letuta, U.G.; Berdinskiy, V.L. Biological effects of static magnetic fields and zinc isotopes on E. coli bacteria. Bioelectromagnetics 2019, 40, 62–73. [Google Scholar] [CrossRef] [Green Version]
- Lysenko, O.B.; Demikhov, Y.N.; Skul’skii, N.A.; Sobotovich, E.V. The role of a magnetic effect in uranium isotope fractionation. Russ. J. Phys. Chem. B 2014, 8, 870–873. [Google Scholar] [CrossRef]
- Sobczyk, L.; Obrzud, M.; Filarowski, A. H/D isotope effects in hydrogen bonded systems. Molecules 2013, 18, 4467–4476. [Google Scholar] [CrossRef] [Green Version]
- Ishikita, H.; Saito, K. Proton transfer reactions and hydrogen-bond networks in protein environments. J. R. Soc. Interface 2014, 11, 20130518. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.-B.; Hu, Q.; Niu, D.-H.; Zheng, X.-H.; Shi, H.-L.; Wang, M.; Xiao, Z.-S.; Huang, A.-P.; Zhang, Z.-B. Research progress of hydrogen tunneling in two-dimensional materials. Acta Phys. Sin. 2017, 66, 056601. [Google Scholar] [CrossRef]
- Drechsel-Grau, C.; Marx, D. Exceptional isotopic-substitution effect: Breakdown of collective proton tunneling in hexagonal ice due to partial deuteration. Angew. Chem. Int. Ed. Engl. 2014, 53, 10937–10940. [Google Scholar] [CrossRef] [PubMed]
- Northrop, D.B. Unusual origins of isotope effects in enzyme-catalysed reactions. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1341–1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uphaus, R.A.; Flaumenhaft, E.; Katz, J.J. A living organism of unusual isotopic composition. Sequential and cumulative replacement of stable isotopes in Chlorella vulgaris. Biochim. Biophys. Acta 1967, 141, 625–632. [Google Scholar] [CrossRef]
- Sarsa, A.; Alcaraz-Pelegrina, J.M.; Le Sech, C. Isotopic effects on covalent bond confined in a penetrable sphere. J. Phys. Chem. B 2015, 119, 14364–14372. [Google Scholar] [CrossRef]
- Karlson, A.M.L.; Reutgard, M.; Garbaras, A.; Gorokhova, E. Isotopic niche reflects stress-induced variability in physiological status. R. Soc. Open Sci. 2018, 5, 171398. [Google Scholar] [CrossRef] [Green Version]
- Symes, C.; Skhosana, F.; Butler, M.; Gardner, B.; Woodborne, S. Isotope (δ13C, δ15N, δ2H) diet–tissue discrimination in African grey parrot Psittacus erithacus: Implications for forensic studies. Isot. Environ. Health Stud. 2017, 53, 580–596. [Google Scholar] [CrossRef]
- Borek, E.; Rittenberg, D. Anomalous growth of microorganisms produced by changes in isotopes in their environment. Proc. Natl. Acad. Sci. USA 1960, 46, 777–782. [Google Scholar] [CrossRef] [Green Version]
- Abeni, F.; Petrera, F.; Capelletti, M.; Dal Prà, A.; Bontempo, L.; Tonon, A.; Camin, F. Hydrogen and oxygen stable isotope fractionation in body fluid compartments of dairy cattle according to season, farm, breed, and reproductive stage. PLoS ONE 2015, 10, e0127391. [Google Scholar] [CrossRef]
- Reynard, L.M.; Burt, N.; Koon, H.E.C.; Tuross, N. Limits and possibilities in the geolocation of humans using multiple isotope ratios (H, O, N, C) of hair from east coast cities of the USA. Isot. Environ. Health Stud. 2016, 52, 498–512. [Google Scholar] [CrossRef] [PubMed]
- Kirsanow, K.; Tuross, N. Oxygen and hydrogen isotopes in rodent tissues: Impact of diet, water and ontogeny. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2011, 310, 9–16. [Google Scholar] [CrossRef]
- Lysenko, O.B.; Skulskiy, N.A.; Shatilo, V.B.; Koshliakova, T.A.; Lysenko, M.O.; Sobotovich, E.V. Problems and prospects of the main biogenic elements intramolecular isotope correlations using as a new resource of different etiology pathologies diagnostics. Zhivye i Biokosnye Sistemy 2014, 8, 1–23. [Google Scholar]
- Fuller, B.T.; Fuller, J.L.; Harris, D.A.; Hedges, R.E. Detection of breastfeeding and weaning in modern human infants with carbon and nitrogen stable isotope ratios. Am. J. Phys. Anthropol. 2006, 129, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Snyder, M.P. Can heavy isotopes increase lifespan? Studies of relative abundance in various organisms reveal chemical perspectives on aging. BioEssays 2016, 38, 1093–1101. [Google Scholar] [CrossRef] [Green Version]
- Gregg, C.T.; Hutson, J.Y.; Prine, J.R.; Ott, D.G.; Furchuer, J.E. Substantial replacement of mammalian body carbon with carbon-13. Life Sci. 1973, 13, 775–782. [Google Scholar] [CrossRef]
- Strable, M.S.; Tschanz, C.L.; Varamini, B.; Chikaraishi, Y.; Ohkouchi, N.; Brenna, J.T. Mammalian DNA δ15N exhibits 40‰ intramolecular variation and is unresponsive to dietary protein level. Rapid Commun. Mass Spectrom. 2011, 25, 3555–3562. [Google Scholar] [CrossRef]
- Felsing, M.; Telfer, T.; Glencross, B. 15N-enrichment of an aquaculture diet and tracing of cage culture waste in an estuarine environment. J. Appl. Ichthyol. 2006, 22, 419–426. [Google Scholar] [CrossRef]
- Crotty, F.V.; Blackshaw, R.P.; Murray, P.J. Tracking the flow of bacterially derived 13C and 15N through soil faunal feeding channels. Rapid Commun. Mass Spectrom. 2011, 25, 1503–1513. [Google Scholar] [CrossRef]
- Gorokhova, E. Shifts in rotifer life history in response to stable isotope enrichment: Testing theories of isotope effects on organismal growth. R. Soc. Open Sci. 2017, 4, 160810. [Google Scholar] [CrossRef] [Green Version]
- Avdeeva, L.V.; Koltover, V.K. Nuclear spin catalysis in living nature. Mosc. Univ. Chem. Bull. 2016, 71, 160–166. [Google Scholar] [CrossRef]
- Buchachenko, A.L. Mass-independent isotope effects. J. Phys. Chem. B 2013, 117, 2231–2238. [Google Scholar] [CrossRef] [PubMed]
- Elharram, A.; Czegledy, N.M.; Golod, M.; Milne, G.L.; Pollock, E.; Bennett, B.M.; Shchepinov, M.S. Deuterium-reinforced polyunsaturated fatty acids improve cognition in a mouse model of sporadic Alzheimer’s disease. FEBS J. 2017, 284, 4083–4095. [Google Scholar] [CrossRef] [PubMed]
- Raefsky, S.M.; Furman, R.; Milne, G.; Pollock, E.; Axelsen, P.; Mattson, M.P.; Shchepinov, M.S. Deuterated polyunsaturated fatty acids reduce brain lipid peroxidation and hippocampal amyloid β-peptide levels, without discernable behavioral effects in an APP/PS1 mutant transgenic mouse model of Alzheimer’s disease. Neurobiol. Aging 2018, 66, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Shchepinov, M.S.; Chou, V.P.; Pollock, E.; Langston, J.W.; Cantor, C.R.; Molinari, R.J.; Manning-Bog, A.B. Isotopic reinforcement of essential polyunsaturated fatty acids diminishes nigrostriatal degeneration in a mouse model of Parkinson’s disease. Toxicol. Lett. 2011, 207, 97–103. [Google Scholar] [CrossRef]
- Zlatska, A.; Gordiienko, I.; Vasyliev, R.; Zubov, D.; Gubar, O.; Rodnichenko, A.; Syroeshkin, A.; Zlatskiy, I. In vitro study of deuterium effect on biological properties of human cultured adipose-derived stem cells. Sci. World J. 2018, 2018, e5454367. [Google Scholar] [CrossRef] [Green Version]
- Dzhimak, S.S.; Basov, A.A.; Fedulova, L.V.; Didikin, A.S.; Bikov, I.M.; Arcybasheva, O.M.; Naumov, G.N.; Baryshev, M.G. Correction of metabolic processes in rats during chronic endotoxicosis using isotope (D/H) exchange reactions. Biol. Bull. 2015, 42, 440–448. [Google Scholar] [CrossRef]
- Andreyev, A.Y.; Tsui, H.S.; Milne, G.L.; Shmanai, V.V.; Bekish, A.V.; Fomich, M.A.; Pham, M.N.; Nong, Y.; Murphy, A.N.; Clarke, C.F.; et al. Isotope-reinforced polyunsaturated fatty acids protect mitochondria from oxidative stress. Free Radic. Biol. Med. 2015, 82, 63–72. [Google Scholar] [CrossRef]
- Hill, S.; Hirano, K.; Shmanai, V.V.; Marbois, B.N.; Vidovic, D.; Bekish, A.V.; Kay, B.; Tse, V.; Fine, J.; Clarke, C.F.; et al. Isotope-reinforced polyunsaturated fatty acids protect yeast cells from oxidative stress. Free Radic. Biol. Med. 2011, 50, 130–138. [Google Scholar] [CrossRef]
- Hill, S.; Lamberson, C.R.; Xu, L.; To, R.; Tsui, H.S.; Shmanai, V.V.; Bekish, A.V.; Awad, A.M.; Marbois, B.N.; Cantor, C.R.; et al. Small amounts of isotope-reinforced polyunsaturated fatty acids suppress lipid autoxidation. Free Radic. Biol. Med. 2012, 53, 893–906. [Google Scholar] [CrossRef] [Green Version]
- Firsov, A.M.; Fomich, M.A.; Bekish, A.V.; Sharko, O.L.; Kotova, E.A.; Saal, H.J.; Vidovic, D.; Shmanai, V.V.; Pratt, D.A.; Antonenko, Y.N.; et al. Threshold protective effect of deuterated polyunsaturated fatty acids on peroxidation of lipid bilayers. FEBS J. 2019, 286, 2099–2117. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin-Chabot, C.; Wang, L.; Smarun, A.V.; Vidovic, D.; Shchepinov, M.S.; Thibault, G. Deuterated polyunsaturated fatty acids reduce oxidative stress and extend the lifespan of C. Elegans. Front. Physiol. 2019, 10, 641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotticelli, M.G.; Crabbe, A.M.; Wilson, R.B.; Shchepinov, M.S. Insights into the role of oxidative stress in the pathology of Friedreich ataxia using peroxidation resistant polyunsaturated fatty acids. Redox. Biol. 2013, 1, 398–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zesiewicz, T.; Heerinckx, F.; De Jager, R.; Omidvar, O.; Kilpatrick, M.; Shaw, J.; Shchepinov, M.S. Randomized, clinical trial of RT001: Early signals of efficacy in Friedreich’s ataxia. Mov. Disord. 2018, 33, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Hatami, A.; Zhu, C.; Relaño-Gines, A.; Elias, C.; Galstyan, A.; Jun, M.; Milne, G.; Cantor, C.R.; Chesselet, M.F.; Shchepinov, M.S. Deuterium-reinforced linoleic acid lowers lipid peroxidation and mitigates cognitive impairment in the Q140 knock in mouse model of Huntington’s disease. FEBS J. 2018, 285, 3002–3012. [Google Scholar] [CrossRef] [PubMed]
- Somlyai, G.; Javaheri, B.; Davari, H.; Gyöngyi, Z.; Somlyai, I.; Tamaddon, K.A.; Boros, L.G. Pre-clinical and clinical data confirm the anticancer effect of deuterium depletion. Biomacromol. J. 2016, 2, 1–7. [Google Scholar] [CrossRef]
- Shikhlyarova, A.I.; Zhukova, G.V.; Dzhimak, S.S.; Baryshev, M.G.; Kit, O.I.; Goncharova, A.S.; Shirnina, E.A.; Protasova, T.P.; Zhadobina, A.I.; Bragina, M.I.; et al. Influence of deuterium-depleted water as a nutritive factor on the effectiveness of experimental chemotherapy in aging rats. J. Clin. Oncol. 2019, 37, e14683. [Google Scholar] [CrossRef]
- Boros, L.G.; Que Collins, T.; Somlyai, G. What to eat or what not to eat—that is still the question. Neuro Oncol. 2017, 19, 595–596. [Google Scholar] [CrossRef] [Green Version]
- Chistyakov, D.V.; Filimonov, I.S.; Azbukina, N.V.; Goriainov, S.V.; Chistyakov, V.V.; Fomich, M.A.; Bekish, A.V.; Shmanai, V.V.; Sergeeva, M.G.; Shchepinov, M.S. Deuterated arachidonic acids library for regulation of inflammation and controlled synthesis of eicosanoids: An in vitro study. Molecules 2018, 23, 3331. [Google Scholar] [CrossRef] [Green Version]
- Yavari, K.; Gholamali, M.; Yazdian, F. The biological effects of deuterium depleted water: A possible tool in diabetes therapy. Electron. J. Biol. 2017, 13, 314–319. [Google Scholar]
- Rehakova, R.; Klimentova, J.; Cebova, M.; Barta, A.; Matuskova, Z.; Labas, P.; Pechanova, O. Effect of deuterium-depleted water on selected cardiometabolic parameters in fructose-treated rats. Physiol. Res. 2016, 65, S401–S407. [Google Scholar] [PubMed]
- Berbee, J.F.P.; Mol, I.M.; Milne, G.L.; Pollock, E.; Hoeke, G.; Lütjohann, D.; Monaco, C.; Rensen, P.C.N.; van der Ploeg, L.H.T.; Shchepinov, M.S. Deuterium-reinforced polyunsaturated fatty acids protect against atherosclerosis by lowering lipid peroxidation and hypercholesterolemia. Atherosclerosis 2017, 264, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Lamberson, C.R.; Xu, L.; Muchalski, H.; Montenegro-Burke, J.R.; Shmanai, V.V.; Bekish, A.V.; McLean, J.A.; Clarke, C.F.; Shchepinov, M.S.; Porter, N.A. Unusual kinetic isotope effects of deuterium reinforced polyunsaturated fatty acids in tocopherol-mediated free radical chain oxidations. J. Am. Chem. Soc. 2014, 136, 838–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korneenko, T.V.; Pestov, N.B.; Hurski, A.L.; Fedarkevich, A.M.; Shmanai, V.V.; Brenna, J.T.; Shchepinov, M.S. A strong developmental isotope effect in Caenorhabditis elegans induced by 5,5-deuterated lysine. Amino Acids 2017, 49, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Pestov, N.B.; Okkelman, I.A.; Shmanai, V.V.; Hurski, A.L.; Giaccia, A.J.; Shchepinov, M.S. Control of lysyl oxidase activity through site-specific deuteration of lysine. Bioorg. Med. Chem. Lett. 2011, 21, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, C.B.; Ulashchik, E.A.; Poopeiko, N.E.; Shmanai, V.V.; Reich, N.O.; Shchepinov, M.S. Rational manipulation of DNA methylation by using isotopically reinforced cytosine. ChembioChem 2016, 17, 2018–2021. [Google Scholar] [CrossRef]
- Lowenson, J.D.; Shmanai, V.V.; Shklyaruck, D.; Clarke, S.G.; Shchepinov, M.S. Deuteration protects asparagine residues against racemization. Amino Acids 2016, 48, 2189–2196. [Google Scholar] [CrossRef] [Green Version]
- Tung, R. The development of deuterium-containing drugs. Innov. Pharm. Technol. 2010, 32, 24–28. [Google Scholar]
- Dzhimak, S.S.; Drobotenko, M.I.; Basov, A.A.; Svidlov, A.A.; Fedulova, L.V.; Lyasota, O.M.; Baryshev, M.G. Mathematical modeling of open states in DNA molecule depending on the deuterium concentration in the surrounding liquid media at different values of hydrogen bond disruption energy. Dokl. Biochem. Biophys. 2018, 483, 359–362. [Google Scholar] [CrossRef]
- Zubarev, R.A. Role of stable isotopes in life—testing isotopic resonance hypothesis. Genom. Proteom. Bioinform. 2011, 9, 15–20. [Google Scholar] [CrossRef] [Green Version]



| I | II | III | IV | V | VI | VII | VIII | IX | X |
|---|---|---|---|---|---|---|---|---|---|
| 12C-1H | 12C-2H | ||||||||
| 6n − 7p | −1 | 7n − 7p | 0 | ||||||
| R-spin | 0+ & ½+ is ½+ + | R-spin | 0+ & 1+ is 1+ + | ||||||
| 13C-1H [84] | 13C-2H [41] | ||||||||
| 7n − 7p | 0 | 8n − 7p | 1 | ||||||
| R-spin | ½− & ½+ is 1− + | R-spin | ½− & 1+ is 1½− + | ||||||
| 14N–1H | 14N-2H | ||||||||
| 7n − 8p | −1 | 8n − 8p | 0 | ||||||
| R-spin | 1+ & ½+ is 1½+ + | R-spin | 1+ & 1+ is 2+ + | ||||||
| 15N-1H [84] | 15N-2H | ||||||||
| 8n − 8p | 0 | 9n − 8p | 1 | ||||||
| R-spin | ½− & ½+ is 1− + | R-spin | ½− & 1+ is 1½− + | ||||||
| 16O-1H | 17O-1H | 18O-1H [84] | |||||||
| 8n − 9p | −1 | 9n − 9p | 0 | 10n − 9p | 1 | ||||
| R-spin | 0+ & ½+ is ½+ + | R-spin | 5/2+ & ½+ is 3+ + | R-spin | 0+ & ½+ is ½+ + | ||||
| 16O-2H | 17O-2H | 18O-2H | |||||||
| 9n − 9p | 0 | 10n − 9p | 1 | 11n − 9p | 2 | ||||
| R-spin | 0+ & 1+ is 1+ + | R-spin | 5/2+ & 1+ is 3½+ + | R-spin | 0+ & 1+ is 1+ + | ||||
| 32S-1H | 32S-2H | 34S-1H | 34S-2H | ||||||
| 16n − 17p | −1 | 17n − 17p | 0 | 18n − 17p | 1 | 19n − 17p | 2 | ||
| R-spin | 0+ & ½+ is ½+ + | R-spin | 0+ & 1+ is 1+ + | R-spin | 0+ & ½+ is ½+ + | R-spin | 0+ & 1+ is 1+ + | ||
| 12C-12C | 12C-13C | ||||||||
| 12n − 12p | 0 | 13n − 12p | 1 | ||||||
| R-spin | 0+ & 0+ is 0+ + | R-spin | 0+ & ½− is ½+ − | ||||||
| 12C-14N | 12C-15N | ||||||||
| 13n − 13p | 0 | 14n − 13p | 1 | ||||||
| R-spin | 0+ & 1+ is 1+ + | R-spin | 0+ & ½− is ½ + − | ||||||
| 13C-14N | 13C-15N [84,85,86] | ||||||||
| 14n − 13p | 1 | 15n − 13p | 2 | ||||||
| R-spin | ½− & 1+ is 1½ + − | R-spin | ½− & ½− is 1 − − | ||||||
| 12C-16О | 12C-17О | 12C-18О | |||||||
| 14n − 14p | 0 | 15n − 14p | 1 | 16n − 14p | 2 | ||||
| R-spin | 0+ & 0+ is 0+ + | R-spin | 0+ & 5/2+ is 5/2+ + | R-spin | 0+ & 0+ is 0+ + | ||||
| 13C-16О | 13C-17О | 13C-18О [84] | |||||||
| 15n − 14p | 1 | 16n − 14p | 2 | 17n − 14p | 3 | ||||
| R-spin | ½− & 0+ is ½− + | R-spin | ½− & 5/2+ is 3− + | R-spin | ½− & 0+ is ½− + | ||||
| 14N-16O | 14N-17O | 14N-18O | |||||||
| 15n − 15p | 0 | 16n − 15p | 1 | 17n − 15p | 2 | ||||
| R-spin | 1+ & 0+ is 1+ + | R-spin | 1+ & 5/2+ is 3½+ + | R-spin | 1+ & 0+ is 1+ + | ||||
| 15N-16O [87] | 15N-17O | 15N-18O [84] | |||||||
| 16n − 15p | 1 | 17n − 15p | 2 | 18n − 15p | 3 | ||||
| R-spin | ½− & 0+ is ½ − + | R-spin | ½− & 5/2+ is 3 − + | R-spin | ½− & 0+ is ½− + | ||||
| 40Ca [27] | 43Ca [27] | ||||||||
| 20n − 20p | 0 | 23n − 20p | 3 | ||||||
| R-spin | 0+ | R-spin | 7/2− | ||||||
| 24Mg [29] | 25Mg [29,88] | 26Mg [29] | |||||||
| 12n − 12p | 0 | 13n − 12p | 1 | 14n − 12p | 2 | ||||
| R-spin | 0+ | R-spin | 5/2+ | R-spin | 0+ | ||||
| 63Cu-18O | |||||||||
| 44n − 37p | 7 | ||||||||
| R-spin | 3/2− & 0+ is 3/2− + | ||||||||
| 63Cu | |||||||||
| 34n − 29p | 5 | ||||||||
| R-spin | 3/2− | ||||||||
| 65Cu | |||||||||
| 36n − 29p | 7 | ||||||||
| R-spin | 3/2− | ||||||||
| 28Si [89] | 29Si [89] | 30Si [89] | |||||||
| 14n − 14p | 0 | 15n − 14p | 1 | 16n − 14p | 2 | ||||
| R-spin | 0+ | R-spin | 1/2+ | R-spin | 0+ | ||||
| 64Zn [90,91] | 67Zn [91,92] | ||||||||
| 34n − 30p | 4 | 37n − 30p | 7 | ||||||
| R-spin | 0+ | R-spin | 5/2− | ||||||
| 66Zn [90,91] | |||||||||
| 36n − 30p | 6 | ||||||||
| R-spin | 0+ | ||||||||
| 238U [93] | 235U [93] | ||||||||
| 146n − 92p | 54 | 143n − 92p | 51 | ||||||
| R-spin | 0+ | R-spin | 7/2− |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basov, A.; Fedulova, L.; Vasilevskaya, E.; Dzhimak, S. Possible Mechanisms of Biological Effects Observed in Living Systems during 2H/1H Isotope Fractionation and Deuterium Interactions with Other Biogenic Isotopes. Molecules 2019, 24, 4101. https://doi.org/10.3390/molecules24224101
Basov A, Fedulova L, Vasilevskaya E, Dzhimak S. Possible Mechanisms of Biological Effects Observed in Living Systems during 2H/1H Isotope Fractionation and Deuterium Interactions with Other Biogenic Isotopes. Molecules. 2019; 24(22):4101. https://doi.org/10.3390/molecules24224101
Chicago/Turabian StyleBasov, Alexander, Liliya Fedulova, Ekaterina Vasilevskaya, and Stepan Dzhimak. 2019. "Possible Mechanisms of Biological Effects Observed in Living Systems during 2H/1H Isotope Fractionation and Deuterium Interactions with Other Biogenic Isotopes" Molecules 24, no. 22: 4101. https://doi.org/10.3390/molecules24224101