Synthesis of Novel UV Absorbers Bisindolylmethanes and Investigation of Their Applications on Cotton-Based Textile Materials
Abstract
:1. Introduction
2. Results and Discussion
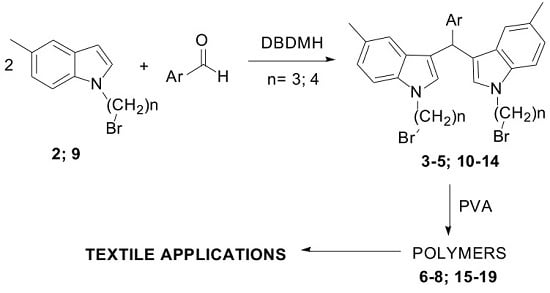
2.1. Synthesis
2.2. Textile Applications
3. Materials and Methods
3.1. General Information
3.2. Synthesis of 2 and 9
3.3. General Procedure of Bromoalkylbis(indolyl)methanes (3–5, 10–14)
3.4. Synthesis of Polymers (6–8, 15–19)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. Environmental Health Criteria 160WHO/EHG/95.16. 2012. Available online: http://www.who.int/uv/publications (accessed on 3 February 2016).
- Matsumura, Y.; Ananthaswamy, H.N. Toxic effects of ultraviolet radiation on the skin. Toxicol. Appl. Pharm. 2004, 195, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, H. Changes of polymer morphology caused by UV irradiation: 2 surface destruction of polymer blends. Polymer 1996, 37, 547–553. [Google Scholar] [CrossRef]
- Kursun, S.; Ozcan, G. An investigation of UV protection of swimwear fabrics. Text. Res. J. 2010, 80, 1811–1818. [Google Scholar] [CrossRef]
- Czajkowski, W.; Paluszkiewicz, P.; Stolarski, R.; Kazmierska, M.; Grzesiak, E. Synthesis of reactive UV absorbers, derivatives of monochlorotriazine, for improvement in protecting properties of cellulose fabrics. Dyes Pigment. 2006, 71, 224–230. [Google Scholar] [CrossRef]
- Rosinskaya, C.; Djani, M.; Kizil, Z.; Weinberg, A. Improvement of UV protection of cotton fabrics dyed with binary mixtures of the reactive dyes. Melliand Int. 2003, 9, 147–148. [Google Scholar]
- Diffey, B.L. Sun protection with closing. Br. J. Dermatol. 2001, 144, 449–450. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Avermaete, A.; Bader, A.; Altmeyer, P.; Hoffman, K. Ultraviolet protection by summer textiles. Ultraviolet transmission measurements verified by determination of the minimal erythema dose with solar-simulated radiation. Br. J. Dermatol. 2001, 144, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Rupp, J.; Hringer, A.B.; Yonenaga, A.; Hilden, J. Textiles for protection against harmful ultraviolet radiation. ITB Int. Text. Bull. 2001, 6, 8–19. [Google Scholar]
- Srinivasan, M.; Gatewood, B. Relationship of dye characteristics to UV protection provided by cotton fabric. Text. Chem. Color Am. Dyest. Rep. 2000, 32, 36–43. [Google Scholar]
- Hironori, O. Development of UV absorbers for sun protective fabrics. Text. Res. J. 2011, 81. [Google Scholar] [CrossRef]
- Akrman, J.; Prikryl, J. Application of benzotriazole reactive UV absorbers to cellulose and determining sun protection of treated fabric spectrophotometrically. J. Appl. Polym. Sci. 2008, 108, 334–341. [Google Scholar] [CrossRef]
- An, Q.F.; Li, L.S.; Lu, D.D. A functional polysiloxane with benzophenone derivative ultraviolet absorbing side groups: Synthesis, morphology, and its performance on fabrics. J. Appl. Polym. Sci. 2007, 104, 680–687. [Google Scholar] [CrossRef]
- Tragoonwichian, S.; O’Rear, E.A.; Yanumet, N. Broad ultraviolet protection by copolymerization of 2-3-(2H-benzotriazol-2-yl)-4-hydroxyphenyl ethyl methacrylate and 2-hydroxy-4-acryloxybenzophenone on cotton via admicellar polymerization. J. Appl. Polym. Sci. 2008, 108, 4004–4013. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X.H.; Li, L. Macromolecular networks assembled by cyclodextrin inclusive associations. Chem. Ind. Eng. Prog. 2008, 27, 1556–1560. [Google Scholar]
- El Tahlawy, K.; El Nagar, K.; Elhendawy, A.G. Cyclodextrin-4 Hydroxybenzophenone inclusion complex for UV protective cotton fabric. J. Text. Inst. 2007, 98, 453–462. [Google Scholar] [CrossRef]
- Uddin, M.J.; Cesano, F.; Bonino, F.; Bordiga, S.; Spoto, G.; Scarano, D.; Zecchina, A. Photoactive TiO2 films on cellulose fibers: Synthesis and characterization. J. Photochem. Photobiol. A Chem. 2007, 189, 286–294. [Google Scholar] [CrossRef]
- Ma, W.; Jiang, X.; Liu, Y.; Tang, B.T.; Zhang, S.F. Synthesis of a novel water-soluble polymeric UV-absorber for cotton. Chin. Chem. Lett. 2011, 22, 1489–1491. [Google Scholar] [CrossRef]
- Cui, Z.H.; Wang, X.D.; Guo, J.C.; Chen, W.G. Synthesis, spectroscopic properties and applications of novel N-heterocycle-containing benzotriazoles as UV absorbers. Chin. Chem. Lett. 2012, 23, 1019–1022. [Google Scholar] [CrossRef]
- Wang, Q.; Hauser, P.J. Developing a novel UV protection process for cotton based on layer-by-layer self-assembly. Carbohydr. Polym. 2010, 81, 491–496. [Google Scholar] [CrossRef]
- Osawa, T.; Namiki, M. Structure elucidation of streptindole, a novel genotoxic metabolite isolated from intestinal bacteria. Tetrahedron Lett. 1983, 24, 4719–4722. [Google Scholar] [CrossRef]
- Zeligs, M.A. Diet and estrogen status: The cruciferous connection. J. Med. Food 1998, 1, 67–82. [Google Scholar] [CrossRef]
- Hojati, S.F.; Zeinali, T.; Nematdoust, Z. A novel method for synthesis of bis(indolyl)methanes using 1,3-Dibromo-5,5-dimethylhydantoin as a highly efficient catalyst under solvent-free conditions. Bull. Korean Chem. Soc. 2013, 34, 117–120. [Google Scholar] [CrossRef]
- Oecotextiles. Available online: https://oecotextiles.wordpress.com/tag/flat-screen-printing/ (accessed on 2 February 2016).
- The American Association of Textile Chemists and Colorists. Transmittance or Blocking of Erythemally Weighted Ultraviolet Radiation through Fabrics; AATCC: Research Triangle Park, NC, USA, 2014. [Google Scholar]
- Standard EN ISO 6330:2012, ISO 6330:2012, Textiles. Domestic washing and drying procedures for textile testing. Available online: https://www.iso.org/obp/ui/#iso:std:43044:en (accessed on 15 March 2015).
- Standard AATCC147-2011, Antibacterial Activity Assessment of Textile Materials. Oekotex 100. Available online: https://www.oeko-tex.com/en/manufacturers/manufacturers.xhtml (accessed on 5 February 2016).
- Sample Availability: Samples of the compounds 2–19 are available from the authors.








| Product | Time (h) | Yield (%) | Mp (°C) | λmax/nm (Absorbance) |
|---|---|---|---|---|
| 2 | 3 | 70 | oil | - |
| 3 | 2 | 55 | 141–142 | 315 (1.52) |
| 4 | 2 | 67 | 118–119 | 310 (1.42) |
| 5 | 2 | 60 | 102 | 300 (1.77) |
| 6 | 3 | 52 | - | 315 (0.60) |
| 7 | 2 | 52 | - | 310 (1.90) |
| 8 | 2 | 47 | - | 315 (1.63) |
| 9 | 2 | 75 | oil | - |
| 10 | 2 | 61 | 127–130 | 290 (1.79) |
| 11 | 2 | 65 | 101–103 | 310 (1.77) |
| 12 | 4 | 72 | 133–135 | 346 (1.60) |
| 13 | 4 | 75 | 168–171 | 296 (1.47) |
| 14 | 4 | 60 | 193–196 | 307 (1.50) |
| 15 | 4 | 65 | - | 283 (1.20) |
| 16 | 4 | 60 | - | 278 (1.00) |
| 17 | 4 | 75 | - | 349 (2.45) |
| 18 | 4 | 70 | - | 300 (0.50) |
| 19 | 4 | 60 | - | 308 (1.22) |
| Polymer | Mw (×105 g/mol) | Mn (×105 g/mol) |
|---|---|---|
| 6 | 5.034 | 4.908 |
| 7 | 5.089 | 4.635 |
| 8 | 5.042 | 4.712 |
| 15 | 15.35 | 15.14 |
| 16 | 11.01 | 10.99 |
| 17 | 10.82 | 7.684 |
| 18 | 8.314 | 7.627 |
| 19 | 5.065 | 4.950 |
| UV Absorber Printed Fabric | UVA Transmittance * (315–400 nm) | UVB Transmittance * (280–315 nm) | UPF | UVA Blocking (%) | UVB Blocking (%) | UPF Label |
|---|---|---|---|---|---|---|
| reference | 5.65 | 2.02 | 33.37 | 94.35 | 97.98 | 27 |
| 6 | 0.30 | 0.10 | 1300.1 | 99.70 | 99.90 | 40+ |
| 7 | 2.15 | 0.22 | 216.77 | 97.85 | 99.78 | 40+ |
| 8 | 3.22 | 0.38 | 146.12 | 96.79 | 99.62 | 40+ |
| 15 | 2.15 | 0.12 | 300.90 | 97.85 | 99.88 | 40+ |
| 16 | 1.47 | 0.10 | 381.05 | 98.53 | 99.90 | 40+ |
| 17 | 0.80 | 0.05 | 1314.43 | 99.20 | 99.95 | 40+ |
| 18 | 1.52 | 0.05 | 594.00 | 98.48 | 99.95 | 40+ |
| 19 | 0.38 | 49.98 | 1604.18 | 99.62 | 99.98 | 40+ |
| Fabric Printed | Washes | Bacteria Growth | Inhibition Zone (mm) * |
|---|---|---|---|
| reference | - | + | |
| 6 | 25 | - | 0 |
| 7 | 25 | - | 0 |
| 8 | 25 | - | 0 |
| 15 | 25 | - | 0 |
| 16 | 25 | - | 0 |
| 17 | 25 | - | 0 |
| 18 | 25 | - | 0 |
| 19 | 25 | - | 0 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ergindemir, H.N.; Aker, A.; Hamitbeyli, A.; Ocal, N. Synthesis of Novel UV Absorbers Bisindolylmethanes and Investigation of Their Applications on Cotton-Based Textile Materials. Molecules 2016, 21, 718. https://doi.org/10.3390/molecules21060718
Ergindemir HN, Aker A, Hamitbeyli A, Ocal N. Synthesis of Novel UV Absorbers Bisindolylmethanes and Investigation of Their Applications on Cotton-Based Textile Materials. Molecules. 2016; 21(6):718. https://doi.org/10.3390/molecules21060718
Chicago/Turabian StyleErgindemir, Hikmet Nil, Acelya Aker, Agamirze Hamitbeyli, and Nuket Ocal. 2016. "Synthesis of Novel UV Absorbers Bisindolylmethanes and Investigation of Their Applications on Cotton-Based Textile Materials" Molecules 21, no. 6: 718. https://doi.org/10.3390/molecules21060718