Alotaibi et al. [
8] utilized a heat exchanger network synthesis where a continuous DSE process was designed and optimized for maximum heat recovery. They confirmed that the DSE process with heat recovery using octanoic acid is more energy efficient when compared to a similar process using decanoic acid. Alotaibi et al. [
8], however, did not consider an exergy analysis as part of their study. Based on their findings, the present study focuses on performing an exergy analysis of the optimized DSE process flow diagram, as shown in
Figure 2.
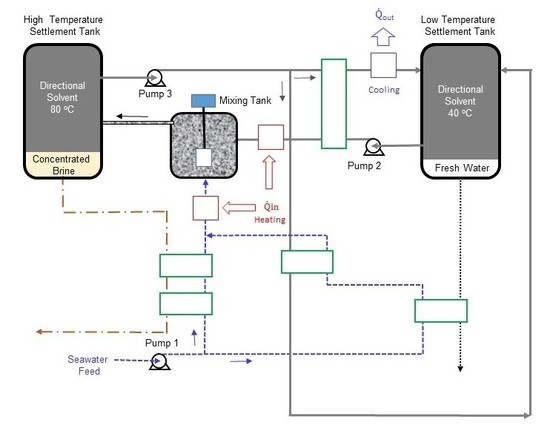
Excluding the complexity of the heat exchanger network, the optimized process mainly consists of a mixing tank, two settlement tanks, and six pumps to circulate the seawater, solvent, and hot water. The basic concept behind this process is that water solubility in the directional solvent, octanoic acid in this case, increases with temperature and the directional solvent has a high rate of salt ion rejection. The process starts in the mixing tank where seawater (State 12) is mixed with the directional solvent (State 8). Before entering the mixing tank, both seawater and solvent are heated to a high temperature, which facilitates water to dissolve into the octanoic acid during mixing. The salt concentration in the brine at State 13 is increased as water is absorbed into the solvent. The solvent saturated with pure water becomes separated from concentrated saline water “brine”, in the high-temperature settlement tank, resulting in two separate streams (States 1 and 14). The saturated solvent (State 1) is pumped through heat exchangers to ultimately reduce its temperature to 40 °C at States 4 and 23. The result of the cooling process is an immiscible mixture of saturated directional solvent and pure water. The solvent and pure water, in the low-temperature settlement tank, are separated into two streams. The purified water stream (State 16) is collected and the saturated solvent stream (State 5) is recycled back to the mixing tank. Four heat exchangers are used to preheat the seawater from State 9 to State 21 and the solvent from State 6 to State 7. Three additional heat exchangers are used for the external heating and cooling processes. One heat exchanger is used to cool the solvent-water mixture (State 3) to the lowest process temperature (State 4). The second heat exchanger is utilized to heat the recycled solvent (State 7) to the highest process temperature (State 8). The third heat exchanger is used to heat the seawater from State 21 to the highest process temperature (State 12). A total of six pumps are used to circulate the solvent, seawater, and hot water in the continuous process.
2.1. Thermodynamic Properties of Seawater
The thermodynamic properties of seawater were evaluated using the modified pressure dependence correlations presented by Nayar et al. [
21], which are based on the previously developed correlations of Sharqawy et al. [
20]. The seawater properties including the specific volume
), enthalpy
, entropy
and chemical potential for salt and water
, respectively, are given as functions of temperature
, pressure
, and salinity
. The seawater flow exergy
is then evaluated by the following equation [
22]:
where
is the mass fraction of salt in seawater, and subscripts “s”, “w” and “sw” refer to salt, water and seawater, respectively.
The thermodynamic properties with superscript “*” represent the restricted state, and are evaluated at temperature and pressure of the global dead state (
and at a salinity of the state (
Sa); while the thermodynamics properties with superscript “
o” are evaluated at
,
, and the salinity of the dead state (
Sao). In this study, the global dead state of seawater is defined as follows:
Equation (1) consists of the thermal and chemical exergies. The thermal exergy is achieved when both temperature and pressure changes to those of the environment with no change in concentration. While the chemical exergy is achieved with a change in concentration, but the temperature and pressure are those of the environment.
2.2. Thermodynamics Properties of Octanoic Acid-Water Binary Mixtures
In the DSE process, there are two binary mixtures. One is the seawater mixture, and the other is the solvent-water mixture. This creates two separate binary mixtures in an immiscible binary system.
Figure 3 below represents the whole system where there are interactions between molecules of water and solvent, and molecules of salt and water, but no interaction between the salt and the solvent. The two separate binary systems at equilibrium allow the evaluation of the seawater properties independently from the solvent-water mixture.
The thermodynamic properties of pure liquid water are evaluated using the correlations by Nayar et al. [
21] at zero salt concentration. For the pure liquid solvent, the specific enthalpy, and entropy are evaluated using the following equations:
where
C is specific heat capacity and
is the specific volume; while subscript “
OA” refers to the octanoic acid solvent and subscript “
o” refers to the reference state. The properties of octanoic acid are given in
Table 2. In this study, the specific heat and specific volume of octanoic acid are assumed constants. The global dead state, which also used as a reference state, of the solvent–water binary mixture is defined as follows:
where
is solubility of water in the directional solvent at the dead state (
The total Gibb’s free energy (
) of the octanoic acid and water binary mixture is given as follows:
where
is the mole fraction the of pure water in the solution,
is Gibb’s free energy of mixing and
is Gibb’s excess energy.
and
are evaluated using the following equations:
The above Gibb’s excess energy equation is based on the two-suffix Margules equation [
23] which is a simple thermodynamic model for Gibb’s excess free energy. Margules equation was used to predict the behavior of non-ideal immiscible liquid binary mixtures, where
A is a constant. For ideal miscible solutions
A = 0; while for non-ideal miscible solutions
A is greater than zero but less than or equal to two (0 <
A ≤2). For non-ideal immiscible solutions,
A is greater than two (
A > 2). For
A ≥ 3, the minimum Gibb’s free energy at equilibrium occurs at low water mole fraction outside the range of the solubility of water in octanoic acid as shown in the shaded area in
Figure 4. For
A = 2.23, however, Gibb’s free energy has a flat minimum that covers the solubility of water in octanoic acid for temperatures between 40 °C to 80 °C, corresponding to a water mole fraction between 0.11 and 0.25. In this study,
A = 2.23 was used to evaluate the thermodynamic properties of the non-ideal octanoic acid–water binary mixtures.
The chemical potential of water (
) and octanoic acid (
) in the solution are evaluated using the following equations:
The enthalpy and entropy of the octanoic acid and water binary mixture,
and
, respectively, are calculated using the following equations:
where
is the mass fraction of pure water in the solution.
The exergy of the octanoic acid-water in the solution
is then evaluated by the following equation:
Similar to Equation (1), the thermodynamic properties with superscript “*” in Equation (11) represent the restricted state, which is evaluated at temperature and pressure of the global dead state ( and at a water solubility of the state (Solu); while the thermodynamics properties with superscript “o” are evaluated at , , and the water solubility of the dead state (Soluo).