1. Introduction
As the global population ages, falls have become an increasingly significant cause of morbidity and mortality in older adults. Around 36 million falls occur among older adults in the United States each year, and 8.4 million of these falls result in serious injuries, such as fracture or head trauma [
1]. Each year, approximately 3 million patients will visit the emergency department for treatment after a fall, many of whom will be hospitalized [
2]. Consequently, the annual total US healthcare cost of falls is around USD 50 billion dollars, with USD 754 million of that spent on fatal falls [
3]. In addition to more acute consequences such as fractures or intracranial bleeds, falls have been associated with diminished quality of life due to increased fear of falling [
4] and remain the leading cause of injury-associated morbidity and mortality among older adults [
5]. Fall risk reduction has long been a topic of geriatric research, but risk factors for falls in patients with known ophthalmologic and neuro-ophthalmologic deficits have been understudied.
Visual impairment is prevalent in around one in four adults aged 71 and older [
6], making it the third-most-common chronic condition in older adults, only following arthritis and heart disease [
7]. The relationship between visual acuity impairment and falls and mortality has been described in a number of cross-sectional and observational cohort studies [
8,
9,
10]. However, fewer studies have examined the relationship of visual impairment to falls in real-world clinical data or in patients with known eye diseases [
11,
12]. To date, there are no studies that parse out the mechanism of falls among patients with known visual impairment due to AION. Most falls are multifactorial, and patients with age-related eye diseases may be systematically different from patients without eye disease, which can also confound the association of vision with fall risk.
The time course of the visual impairment may also complicate the relationship between vision and falls. Most age-related eye diseases progress gradually over time, which presents a challenge when evaluating the temporal impact of vision on fall risk. Patients affected by such slowly progressive age-related eye diseases may accommodate their activity level in response to the gradual change in their vision over time or may reduce their physical activity due to increased fear of falling [
8,
12,
13,
14], which may attenuate the relationship between their level of visual dysfunction and falls. Thus, it may be easier to evaluate the effect of vision on fall risk in patients with acute-onset visual impairment, from causes such as ischemic optic neuropathy, since they will be less likely to have modified their activity level over time.
Thus, in this study, we sought to examine the relationship of vision with fall risk and mortality among patients who experienced a sudden and profound change in vision due to anterior ischemic optic neuropathy (AION). AION is an acute ischemic event of the anterior portion of the optic nerve that causes sudden and severe vision loss and visual field constriction. AION and falls also share many risk factors (i.e., cardiovascular disease, cerebrovascular disease, diabetes, hypertension, etc.). Given its acute-onset, we hypothesized that patients with worse acuity from AION, especially legal blindness, would be at greater risk of falls and mortality than patients with better visual function after AION, and we controlled for other a priori confounders that can be related to falls to attempt to better isolate this association.
2. Materials and Methods
This was a retrospective electronic medical record (EMR) study of adult patients (age > 18 years) presenting with a new diagnosis of AION for ophthalmic evaluation at a single tertiary center from January 2015 to December 2019 with follow-up through February 2023. The Wake Forest University Institutional Review Board approved this study with a waiver of informed consent due to the retrospective nature of this investigation. All methods adhered to the tenets of the Declaration of Helsinki for research involving human subjects and were conducted in accordance with regulations of the Health Insurance Portability and Accountability Act.
2.1. Participant Selection
Patients were initially identified for this study via an Informatics for Integrating Biology and the Bedside (I2B2) query of all Wake Forest Baptist Health patients in the Translational Data Warehouse who received a new diagnosis of anterior ischemic optic neuropathy International Classification of Diseases (ICD) code between 1 January 2015 and 31 December 2019 (
n = 656). Patient cases were drawn from emergency room, inpatient, and outpatient visits. Clinical information from baseline and follow-up visits was reviewed in the EMR, including patient ICD-9/10 codes, demographics (age, sex, race, etc.), medical history and problem lists, medical notes, best-corrected visual acuity, slit-lamp and dilated fundus examinations, and any available relevant imaging, such as stereoscopic optic disc photographs, optical coherence tomography, and 30-2 Humphrey Visual Field scans (Carl Zeiss Meditec, Inc., Dublin, CA, USA). In order to be retained in the study, the patient needed to meet clinical criteria for AION diagnosis, which is sudden painless vision loss with optic nerve head edema. Cases were further classified as arteritic AION if caused by temporal (or giant cell) arteritis, and if associated with typical signs and symptoms in the notes, such as temporal head aches and scalp tenderness, jaw claudication, polymyalgia rheumatica, and elevated inflammatory markers (i.e., erythrocyte sedimentation rate, C reactive protein, and platelet count). Non-arteritic AION was a diagnosis of exclusion after ruling out arteritic AION and other causes of optic nerve edema, such as inflammatory optic neuritis, compressive optic nerve edema (e.g., tumor), posterior ischemic optic neuropathy, central retinal vein occlusion, and central retinal artery occlusion [
15]. Overall, 228 patients were excluded due to diagnosis of a non-anterior ischemic optic neuropathy (e.g., compressive optic neuropathy), a historic optic neuropathy diagnosis prior to initial visit, any diagnosis of glaucoma/glaucoma suspect, or if there were no eye exam data available. There were no exclusions made solely on the basis of age, sex, race, socioeconomic status, insurance status, employment status, or other systemic comorbidities. After exclusions, the final sample included 428 patients.
2.2. Data Collection
The dates of new AION diagnosis, incident EMR encounter for a fall, and date of death were exported from I2B2 and were manually confirmed in the EMR. Patient visual acuity (VA) from the initial exam date with AION was converted to the logarithm of the minimum angle of resolution (logMAR) and was also further categorized as legal blindness or not in the affected eye (20/200 or worse). Automated perimetry was available on a subset of patients (30-2 Sita-Standard Humphrey visual fields, Zeiss Meditec, Inc.), and the mean deviation was collected from the visual field report. The visual fields were also reviewed and, for the purpose of analyses, were classified into global constriction versus less than global field loss (i.e., inferior, superior, central scotoma, etc.). Additional details about the AION were also collected but are not presented in this analysis since there was a lack of power to investigate these further (i.e., whether AION was arteritic or non-arteritic, incident fractures, subsequent hospitalizations or surgeries as the result of fall).
A priori confounding risk factors were also obtained by a careful review of patient notes, history, problem lists, and medications and included the following: hypertension (HTN), diabetes mellitus (DM), any diagnosis consistent with dementia (e.g., Alzheimer’s disease, frontotemporal dementia, etc.), cardiovascular disease (i.e., history of any myocardial infarction, congestive heart failure, coronary artery disease, cardiothoracic surgery, peripheral artery disease, congenital heart malformations, atrial fibrillation, other arrhythmias, etc.), cerebrovascular disease (i.e., stroke), and polypharmacy (i.e., taking 5 or more systemic medications). Also, the presence of other common age-related eye conditions in either eye (i.e., cataracts, diabetic retinopathy, age-related macular degeneration (ARMD)) was noted to include in the analysis.
2.3. Data Analysis
The sample baseline clinical and demographic characteristics were described using descriptive statistics. Separate bivariate Cox proportional hazards models were constructed to demonstrate the relationship of each clinical and demographic factor with survival time to (1) first fall and (2) mortality. In addition, separate bivariate Cox proportional hazards models were constructed to evaluate the relationship of each visual acuity measure—logMAR visual acuity (VA) and legal blindness (20/200 or worse VA)—in the affected eye at time of AION diagnosis and survival time to (1) first fall, (2) mortality, and (3) a composite outcome of fall or death. For models of fall risk, the outcome was the first fall, and patients were otherwise censored at the date of death or date lost to follow-up. For models of mortality, the outcome was death, and patients were otherwise censored at the date lost to follow-up. Separate multivariable Cox proportional hazards models focused on the relationship of vision (logMAR VA or legal blindness) with (1) incident fall, (2) mortality, or (3) the composite outcome while adjusting for all a priori confounders (age, sex, race, ophthalmologic comorbidities (cataracts, age-related macular degeneration, diabetic retinopathy), and systemic comorbidities (hypertension, diabetes mellitus, cardiovascular disease, cerebrovascular disease, dementia, and polypharmacy). In a subset with reliable visual field data, separate unadjusted and adjusted Cox proportional hazards models were constructed to evaluate the relationship between either MD or visual field pattern (global constriction vs. less than global constriction) and survival time to (1) first fall and (2) mortality. All analyses were conducted in Stata MP (vs. 18.0, StataCorp, College Station, TX, USA). A p-value < 0.05 was considered statistically significant.
3. Results
From the 428 patients with a diagnosis of AION meeting study criteria, 53 had at least one documented fall after their AION diagnosis, and 56 died during the study period.
Table 1 describes the baseline characteristics of these 428 subjects and the unadjusted incidence of falls and mortality for each demographic or clinical factor. The average age of the study population was 68.1 ± 12.3 years, which is consistent with the mean age of onset for AION of 62–65 [
15,
16]. There were slightly fewer females (40.4%) than males. Cumulatively, over half of the subjects had underlying eye conditions comorbid to their AION (47.4% with active cataract in at least one eye, 12.4% with age-related macular degeneration, and 11.7% with diabetic retinopathy). The majority (83.4%) of the subjects were on five or more systemic medications, consistent with polypharmacy [
17]. In unadjusted models, older age, non-white race, cardiovascular disease, cerebrovascular disease, hypertension, and dementia were associated with an increased risk of incident falls (all
p < 0.05). Patients with cataracts were less likely to experience a fall. Older age, cardiovascular disease, cerebrovascular disease, and both visual acuity and legal blindness (VA 20/200 or worse) were predictors of mortality (all
p < 0.05).
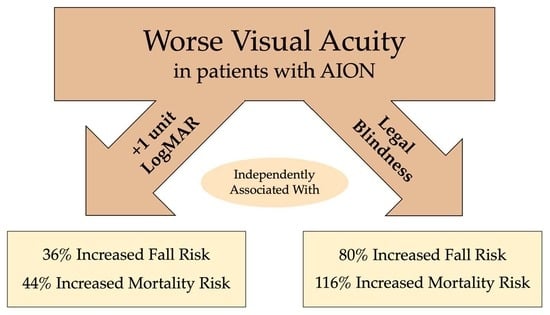
In multivariable models that adjusted for a priori ophthalmic, demographic, and clinical confounders, worse logMAR VA and legal blindness (best-corrected vision of 20/200 or worse) were both associated with increased incident fall risk and mortality risk among patients with a new diagnosis of AION (
Table 2,
Supplemental Table S1, and
Figure 1). For example, a +1-unit increase in logMAR VA was associated with a 36% greater risk of falls (hazard ratio (HR) 1.36, 95% CI (1.06–1.73),
p = 0.014) and 44% increased risk of mortality (HR 1.44, 95% CI (1.15–1.82),
p = 0.002). Also, there was a greater risk of experiencing either an adverse outcome of an incident fall or death (HR 1.37, 95% CI (1.15–1.62),
p = 0.001). Similarly, in adjusted models (
Table 2,
Supplemental Table S1,
Figure 1), legal blindness was associated with an 80% greater risk of falls (HR 1.80, 95% CI (1.05–3.07),
p = 0.032), a two-fold greater risk of mortality (HR 2.16, 95% CI (1.29–3.63),
p = 0.004), and a 74% increased risk of either adverse outcome (HR 1.74, 95% CI (1.17–2.58),
p = 0.006).
Reliable automated perimetry was available for grading in a subset of cases (n = 216). A better (less negative) mean deviation was associated with a slightly lower fall risk (HR 0.96, 95% CI (0.93, 0.99), p = 0.0.044) in unadjusted Cox proportional hazards model, but this was not significant after adjusting for the other clinical and demographic covariates in multivariable analyses (HR 0.97, 95% CI (0.93, 1.0), p = 0.066). Differences in the time to fall for those with a globally constricted visual field vs. more focal visual field deficit were not significant (unadjusted HR 1.66, 95% CI (0.87, 3.14), p = 0.12; adjusted HR 1.60, 95% CI (0.81, 3.17), p = 0.176). There was no difference in the risk of mortality based on MD (unadjusted HR 1.0, 95% CI (0.96, 1.04), p = 0.91; adjusted HR 1.0, 95% CI (0.97, 1.05), p = 0.70) or having a globally constricted visual field pattern (unadjusted HR 1.06, (95% CI 0.51, 2.18), p = 0.88; adjusted HR 0.86, 95% CI (0.39, 1.90), p = 0.70).
4. Discussion
In this study, we demonstrated that among patients with acute AION, worse visual acuity, especially legal blindness, was associated with a significantly greater risk of incident EMR encounter for a fall and higher risk of mortality. Our study findings underscore the contribution of vision to risk of falls and mortality independent of other systemic conditions, like cerebrovascular and cardiovascular disease, diabetes, hypertension, dementia, and polypharmacy. These results are also remarkable because all included patients had AION and, thus, shared many of the cardiovascular and other risk factors and characteristics that predispose one to incident AION or fall.
Prior studies have used an array of different study designs and highly variable definitions of visual impairment when examining adverse outcomes such as falls and mortality. The majority of studies have utilized cross-sectional design or self-report of prior falls and associated this with the current level of visual impairment, demonstrating that older adults with worse vision are more likely to self-report a prior fall [
8,
9,
13]. For example, the Blue Mountain Eye Study found that only visual acuity and contrast sensitivity were associated with self-report of two or more falls in the prior 12 months per 1 standard deviation decrease in vision in multivariable models [
9]. In terms of other eye conditions, several studies have evaluated fall risk, especially among glaucoma patients. For example, a 2021 cross-sectional study by Bhorade et al. found that glaucoma patients were more likely to self-report a fall in the prior 12 months compared to control subjects [
14]. However, causal inference was limited by the cross-sectional design of these studies. A more recent 2023 meta-analysis exploring 31 articles and nearly 71,000 senior patients in China by Li et al. determined that “unclear vision” (which was defined differently in each of the five studies analyzed) was a significant risk factors for falls (HR 1.56) [
18]. The effect size is similar to our study, which found an adjusted HR of 1.36 for every 1-unit logMAR increase and an HR of 1.80 for legal blindness. However, others have produced less conclusive findings, with some failing to find any association with visual acuity in adjusted analyses. For example, the Salisbury Eye Study was a prospective cohort study, which observed a very small increase in the odds of falls over 20 months among older adults with worse visual fields but did not detect a relationship with visual acuity after adjusting for demographics and patient health [
19].
The differences in study findings could be related to differences in the study design (cross-sectional vs. longitudinal) as well as differences in the definition of the fall outcome (self-reported history of falls vs. prospective fall surveillance vs. EMR-related incident falls). Moreover, there is likely a more limited range of visual dysfunction in population-based research studies as such studies tend to enroll healthier patients with better vision than the general population, compared to studies of vision in patients with known eye diseases or in real-world clinical data. Our study examined patients who had experienced an acute ischemic event known to impact visual function, and, thus, the patients who fell and who did not fall shared a lot of other clinical characteristics, which helped to limit confounding. We were also careful to control for a number of a priori conditions that have been associated with falls so we could better isolate vision. Moreover, our patient cohort was drawn from real-world clinical data, and the outcome of falls that resulted in a healthcare-related encounter has particular relevance to healthcare costs.
Visual impairment was also highlighted as a potentially modifiable risk factor for mortality in a 2021 meta-analysis by Ehrlich et al. (HR 1.29) [
20]. Another study by McCarty et al. suggested that in a cohort of patients over 40 years old in the Melbourne Visual Impairment Project (VIP) database, there was a significantly increased likelihood of mortality (OR 2.34) within 5-year follow-up following enrollment into this project and database [
21]. Such findings are similar to what we observed, in which patients with AION who had worse logMAR visual acuity (HR 1.44) or legal blindness (HR 2.16) were more likely to experience incident mortality, independent of other comorbidities. Similar to falls, the reasons that patients with worse visual dysfunction are predisposed to an earlier death are likely multifactorial, but it is plausible that visual impairment is on the causal pathway since poor vision limits one’s ability to manage other health conditions if one cannot see well enough to read one’s medicine bottles or if one can no longer drive to appointments. In fact, dependence on a driver has been a limiting factor in maintaining good adherence to scheduled visits for the care of multiple chronic eye diseases [
22], and thus likely impacts the patient’s ability to adhere to visits for other systemic conditions as well.
While our findings highlight the role of vision with respect to fall risk in patients with AION, other factors, such as age, non-white race, and cardiovascular disease, also contributed significantly to both fall risk and mortality. Adults with any type of AION may already be at a higher risk of falls, given their older age. Furthermore, those with non-arteritic AION in particular share many vascular risk factors with other older adults who fall, such as diabetes, hypertension, and related cardiovascular and cerebrovascular disease. In our cohort, cardiovascular disease remained significantly associated with both fall risk and mortality in the multivariable models, thus underscoring the important connection of shared vascular risk factors to these outcomes.
Non-white patients were also predisposed to higher fall risk and mortality, which may highlight a potential racial disparity that was not completely explained by the other clinical and demographic covariates in the adjusted models. Racial disparities have been widely documented across the fields of medicine and could be due to differences in access to care and other socioeconomic variance [
23,
24]. This finding also warrants further investigation, as others have found conflicting data on the relationship of race to falls [
25], with some studies suggesting white people are at a greater risk of falls than black people [
26,
27,
28,
29,
30,
31]. However, many of these studies relied on self-reported recall of prior falls, which could be impacted by recall bias. A recent prospective study of white and black people in Boston assessed the rate of falls with prospective calendars and found that most of the difference in fall rates was due to confounding by individual- and community-level characteristics [
25].
Another classic risk factor for NAION is nocturnal hypotension, which may be related to the administration of antihypertensives before bedtime [
32]. While nocturnal hypotension in particular has not been related to falls, polypharmacy has been [
33], and it is possible that patients who are over-medicated with multiple antihypertensive medicines could be less likely to perfuse not only their optic nerve but also their brain, leading to syncopal and stroke-related falls. We did not observe a significant relationship of polypharmacy to falls in our cohort, but the timing of antihypertensive administration or evidence of nocturnal hypotension were not available in this retrospective review. Such factors could be relevant for future investigations. Patients with arteritic AION due to vasculitis of the temporal artery can also present with polymyalgia rheumatica and fatigue, which could predispose to fall risk. However, only 5% of patients in this cohort had temporal arteritis, so we were not powered to study whether the arteritic subtype had a stronger relationship to falls or investigate the role of muscular pain or fatigue. Our study also showed that dementia was another risk group more likely to experience a fall in our study population, but dementia alone was not a significant risk factor for incident mortality. Dementia is not a known risk factor related to AION though vascular contributions to dementia and AION could be shared.
There are limitations in this study. As a single-center retrospective cohort study, the sample size is limited, and we were only able to collect the information available in the electronic medical records within a single health system. It is possible some patients may have presented elsewhere for a fall. This is also not generalizable to falls that occur in the home but that do not result in a health system encounter. Thus, we likely underestimated total falls within our sample during the study window. However, injurious falls which result in a healthcare encounter generate higher healthcare costs and are likely to be more serious. Our analysis of visual field data was also limited by what was available in the medical record. Nearly 40% of patients were 20/200 or worse in the eye affected by AION, which precluded the ability to complete automated perimetry. Also, some patients were evaluated in the emergency room and did not undergo this outpatient procedure. Thus, in many cases, only confrontational visual fields were examined at the time of evaluation, which limited what analysis was feasible with the available perimetry. Of note, while mean deviation was associated with fall risk, this was not significant in the multivariable model. We also did not observe that the defect location was significantly associated with fall risk or mortality, though we may have been underpowered. A prior study of glaucoma patients observed that worse integrated visual field sensitivity was more strongly associated with fall-relevant gait features than either inferior or superior defects [
34]. Inferior visual field loss has been associated with greater fear of falling in glaucoma [
35] and also higher fall risk in glaucoma [
36], but those studies did not consider acuity, which is often preserved until the very end stage of disease. We excluded patients with glaucoma since this could confound the ability to distinguish AION, and we also controlled for common co-existent ocular pathology in either eye (such as cataract, age-related macular degeneration, or diabetic retinopathy). In our cohort, we may not have been powered to find differences based on visual field loss due to the more limited sample size. However, central acuity loss was a significant factor related to falls and mortality and was available for all included patients. Other types of visual function that could be important for falls, such as contrast sensitivity, were not available in the medical record since they are not routinely collected in clinical care. One unexpected finding was that patients with cataracts were less likely to experience a fall. We suspect that this was confounded in part by age if patients who were younger were more likely to be phakic and not have undergone cataract surgery yet.
There are also notable strengths to this study. While our initial query was based on ICD-10 codes for ischemic optic neuropathy, we manually reviewed each patient’s medical record notes, exam data, and imaging to confirm the diagnosis, event timing, and comorbidities. This helped us ensure we included definite new diagnoses of AION and excluded other types of optic neuropathy. Moreover, we controlled for a number of other a priori risk factors for falls in order to better quantify the relationship of vision to fall risk and mortality, with an emphasis on potentially shared vascular risk factors. However, we did not collect for some other potential causes of fall, which we did expect to be associated with both AION and falls (e.g., Meniere’s disease, Parkinson’s disease, hypotension, or seizures). Unmeasured confounding could, thus, explain some of the association we observed.