A Zoonotic Strain of Rocahepevirus ratti Hepatitis E Virus Does Not Replicate Efficiently within Human Placental JEG-3 Cells
Abstract
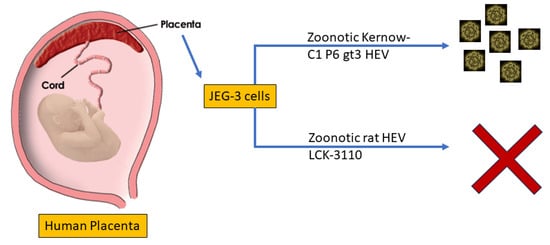
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Linearization of Plasmid DNA
2.3. Proteinase K Digestion and Purification of Linear DNA
2.4. In Vitro Transcription
2.5. Transfection of JEG-3 Cells
2.6. Infectivity Assay
2.7. Flow Cytometry of In Vitro-Transcribed Capped Paslahepevirus balayani (Kernow-C1 P6), Avihepevirus magniiecur, Rocahepevirus ratti HEV RNA Transfected JEG-3 Cells, and Infected HEPG2/C3A Cells
2.8. Immunofluorescence Assay
2.9. Inoculation of Cell Culture
2.10. RNA Extraction and RT-qPCR
- Pasla: HEV F, 5′-GGTGGTTTCTGGGGTGAC-3′, HEV R, 5′-AGGGGTTGGTTGGATGAA-3′, and a probe 5′-FAM-TGATTCTCAGCCCTTCGC-Dabcyl-3′;
- Avi: Avian HEV F, 5′-AATGTGCTGCGGGGTGTCAA-3′, Avian HEV R, 5′-CATCTGG-TACCGTGCGAGTA-3′, and a probe 5′-FAM-CTCCCAAACGCTCCCAGCCGG A-Dabcyl-3′;
- Roca: Rat HEV F, 5′-CTTGTTGAGCTYTTCTCCCCT-3′, Rat HEV R, 5′-CTGTACCGGATGCGACCAA-3′, and a probe 5′-FAM-TGCAGCTTGTCTTTGARCCC-Dabcyl-3′.
2.11. Statistical Analysis and Reproducibility
3. Results
3.1. JEG-3 Is Not Permissive to Rat HEV Replication
3.2. No Detectable Infection of Avian or Rat HEV in HEPG2/C3A Cells Using Transfected JEG-3 Cell Lysates
3.3. Rat HEV LCK-3110 Does Not Replicate in JEG-3 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lhomme, S.; Marion, O.; Abravanel, F.; Izopet, J.; Kamar, N. Clinical Manifestations, Pathogenesis and Treatment of Hepatitis E Virus Infections. J. Clin. Med. 2020, 9, 331. [Google Scholar] [CrossRef] [PubMed]
- Kenney, S.P.; Meng, X.J. Hepatitis E Virus Genome Structure and Replication Strategy. Cold Spring Harb. Perspect. Med. 2019, 9, a031724. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Wang, Y. Transmission of Hepatitis E Virus. Adv. Exp. Med. Biol. 2016, 948, 89–112. [Google Scholar] [CrossRef] [PubMed]
- Ticehurst, J.R.; Pisanic, N.; Forman, M.S.; Ordak, C.; Heaney, C.D.; Ong, E.; Linnen, J.M.; Ness, P.M.; Guo, N.; Shan, H.; et al. Probable transmission of hepatitis E virus (HEV) via transfusion in the United States. Transfusion 2019, 59, 1024–1034. [Google Scholar] [CrossRef] [PubMed]
- Mateos, M.L.; Camarero, C.; Lasa, E.; Teruel, J.L.; Mir, N.; Baquero, F. Hepatitis E virus: Relevance in blood donors and other risk groups. Vox Sang. 1998, 75, 267–269. [Google Scholar] [CrossRef]
- Harvala, H.; Hewitt, P.E.; Reynolds, C.; Pearson, C.; Haywood, B.; Tettmar, K.I.; Ushiro-Lumb, I.; Brailsford, S.R.; Tedder, R.; Ijaz, S. Hepatitis E virus in blood donors in England, 2016 to 2017: From selective to universal screening. Eurosurveillance 2019, 24, 1800386. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Bu, Q.; Gong, W.; Li, H.; Wang, L.; Li, S.; Sridhar, S.; Cy Woo, P.; Wang, L. Hepatitis E virus infection and its associated adverse feto-maternal outcomes among pregnant women in Qinhuangdao, China. J. Matern. Fetal Neonatal Med. 2020, 33, 3647–3651. [Google Scholar] [CrossRef] [PubMed]
- Jilani, N.; Das, B.C.; Husain, S.A.; Baweja, U.K.; Chattopadhya, D.; Gupta, R.K.; Sardana, S.; Kar, P. Hepatitis E virus infection and fulminant hepatic failure during pregnancy. J. Gastroenterol. Hepatol. 2007, 22, 676–682. [Google Scholar] [CrossRef]
- Si, F.; Widen, F.; Dong, S.; Li, Z. Hepatitis E Virus. In Hepatitis E Virus. Advances in Experimental Medicine and Biology; Wang, Y., Ed.; Springer: Singapore, 2023; Volume 1417, pp. 1–13. [Google Scholar]
- Purdy, M.A.; Drexler, J.F.; Meng, X.-J.; Norder, H.; Okamoto, H.; Van der Poel, W.H.; Reuter, G.; de Souza, W.M.; Ulrich, R.G.; Smith, D.B. ICTV virus taxonomy profile: Hepeviridae 2022. J. Gen. Virol. 2022, 103, 001778. [Google Scholar] [CrossRef] [PubMed]
- Johne, R.; Plenge-Bönig, A.; Hess, M.; Ulrich, R.G.; Reetz, J.; Schielke, A. Detection of a novel hepatitis E-like virus in faeces of wild rats using a nested broad-spectrum RT-PCR. J. Gen. Virol. 2010, 91, 750–758. [Google Scholar] [CrossRef]
- Johne, R.; Dremsek, P.; Kindler, E.; Schielke, A.; Plenge-Bönig, A.; Gregersen, H.; Wessels, U.; Schmidt, K.; Rietschel, W.; Groschup, M.H.; et al. Rat hepatitis E virus: Geographical clustering within Germany and serological detection in wild Norway rats (Rattus norvegicus). Infect. Genet. Evol. 2012, 12, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Li, T.C.; Yoshimatsu, K.; Yasuda, S.P.; Arikawa, J.; Koma, T.; Kataoka, M.; Ami, Y.; Suzaki, Y.; Mai, L.T.Q.; Hoa, N.T.; et al. Characterization of self-assembled virus-like particles of rat hepatitis E virus generated by recombinant baculoviruses. J. Gen. Virol. 2011, 92, 2830–2837. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Kunita, S.; Kawakami, M.; Kadosaka, T.; Fujita, H.; Takada, N.; Miyake, M.; Kobayashi, T.; Ohnishi, H.; Nagashima, S.; et al. First detection and characterization of rat hepatitis E Virus (HEV-C1) in Japan. Virus Res. 2022, 314, 198766. [Google Scholar] [CrossRef] [PubMed]
- Purcell, R.H.; Engle, R.E.; Rood, M.P.; Kabrane-Lazizi, Y.; Nguyen, H.T.; Govindarajan, S.; St Claire, M.; Emerson, S.U. Hepatitis E virus in rats, Los Angeles, California, USA. Emerg. Infect. Dis. 2011, 17, 2216–2222. [Google Scholar] [CrossRef] [PubMed]
- Cossaboom, C.M.; Córdoba, L.; Sanford, B.J.; Piñeyro, P.; Kenney, S.P.; Dryman, B.A.; Wang, Y.; Meng, X.J. Cross-species infection of pigs with a novel rabbit, but not rat, strain of hepatitis E virus isolated in the United States. J. Gen. Virol. 2012, 93, 1687–1695. [Google Scholar] [CrossRef]
- Sridhar, S.; Yip, C.C.Y.; Wu, S.; Cai, J.; Zhang, A.J.; Leung, K.H.; Chung, T.W.H.; Chan, J.F.W.; Chan, W.M.; Teng, J.L.L.; et al. Rat Hepatitis E Virus as Cause of Persistent Hepatitis after Liver Transplant. Emerg. Infect. Dis. 2018, 24, 2241–2250. [Google Scholar] [CrossRef] [PubMed]
- Andonov, A.; Robbins, M.; Borlang, J.; Cao, J.; Hatchette, T.; Stueck, A.; Deschambault, Y.; Murnaghan, K.; Varga, J.; Johnston, L. Rat Hepatitis E Virus Linked to Severe Acute Hepatitis in an Immunocompetent Patient. J. Infect. Dis. 2019, 220, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Khuroo, M.S. Hepatitis E and Pregnancy: An Unholy Alliance Unmasked from Kashmir, India. Viruses 2021, 13, 1329. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Li, T.; Xia, Y.; Cong, C.; Chen, S.; Zhang, Y.; Gong, S.; Wang, W.; Liu, H.; Chen, D.; et al. Genotype 4 Hepatitis E virus replicates in the placenta, causes severe histopathological damage, and vertically transmits to fetuses. J. Infect. 2023, 87, 34–45. [Google Scholar] [CrossRef]
- Yadav, K.K.; Kenney, S.P. Hepatitis E Virus Zoonotic Axis. In Zoonoses: Infections Affecting Humans and Animals; Sing, A., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–28. [Google Scholar] [CrossRef]
- Yadav, K.K.; Kenney, S.P. Animal Models for Studying Congenital Transmission of Hepatitis E Virus. Microorganisms 2023, 11, 618. [Google Scholar] [CrossRef]
- Nair, V.P.; Anang, S.; Subramani, C.; Madhvi, A.; Bakshi, K.; Srivastava, A.; Shalimar Nayak, B.; Ranjith Kumar, C.T.; Surjit, M. Endoplasmic Reticulum Stress Induced Synthesis of a Novel Viral Factor Mediates Efficient Replication of Genotype-1 Hepa-titis E Virus. PLoS Pathog. 2016, 12, e1005521. [Google Scholar] [CrossRef] [PubMed]
- Yadav, K.K.; Boley, P.A.; Fritts, Z.; Kenney, S.P. Ectopic Expression of Genotype 1 Hepatitis E Virus ORF4 Increases Genotype 3 HEV Viral Replication in Cell Culture. Viruses 2021, 13, 75. [Google Scholar] [CrossRef] [PubMed]
- Gouilly, J.; Chen, Q.; Siewiera, J.; Cartron, G.; Levy, C.; Dubois, M.; Al-Daccak, R.; Izopet, J.; Jabrane-Ferrat, N.; El Costa, H. Genotype specific pathogenicity of hepatitis E virus at the human maternal-fetal interface. Nat. Commun. 2018, 9, 4748. [Google Scholar] [CrossRef]
- El-Mokhtan El-Mokhtar, M.A.; Othman, E.R.; Khashbah, M.Y.; Ismael, A.; Ghaliony, M.A.; Seddik, M.I.; Sayed, I.M. Evidence of the Extrahepatic Replication of Hepatitis E Virus in Human Endometrial Stromal Cells. Pathogens 2020, 9, 295. [Google Scholar] [CrossRef] [PubMed]
- Knegendorf, L.; Drave, S.A.; Thi, V.L.D.; Debing, Y.; Brown, R.J.; Vondran, F.W.; Resner, K.; Friesland, M.; Khera, T.; Engelmann, M. Hepatitis E virus replication and interferon responses in human placental cells. Hepatol. Commun. 2018, 2, 173–187. [Google Scholar] [CrossRef]
- Ratho, R.K.; Thakur, V.; Arya, S.; Singh, M.P.; Suri, V.; Das, A. Placenta as a site of HEV replication and inflammatory cytokines modulating the immunopathogenesis of HEV in pregnant women. J. Med. Virol. 2022, 94, 3457–3463. [Google Scholar] [CrossRef] [PubMed]
- Yadav, K.K.; Boley, P.A.; Lee, C.M.; Khatiwada, S.; Jung, K.; Laocharoensuk, T.; Hofstetter, J.; Wood, R.; Hanson, J.; Kenney, S.P. Rat hepatitis E virus (HEV) cross-species infection and transmission in pigs. bioRxiv 2023. [Google Scholar] [CrossRef]
- Graff, J.; Torian, U.; Nguyen, H.; Emerson, S.U. A bicistronic subgenomic mRNA encodes both the ORF2 and ORF3 proteins of hepatitis E virus. J. Virol. 2006, 80, 5919–5926. [Google Scholar] [CrossRef]
- Khuroo, M.S.; Kamili, S.; Khuroo, M.S. Clinical course and duration of viremia in vertically transmitted hepatitis E virus (HEV) infection in babies born to HEV-infected mothers. J. Viral Hepat. 2009, 16, 519–523. [Google Scholar] [CrossRef]
- Khuroo, M.S.; Teli, M.R.; Skidmore, S.; Sofi, M.A.; Khuroo, M.I. Incidence and severity of viral hepatitis in pregnancy. Am. J. Med. 1981, 70, 252–255. [Google Scholar] [CrossRef]
- Kumar Acharya, S.; Kumar Sharma, P.; Singh, R.; Kumar Mohanty, S.; Madan, K.; Kumar Jha, J.; Kumar Panda, S. Hepatitis E virus (HEV) infection in patients with cirrhosis is associated with rapid decompensation and death. J. Hepatol. 2007, 46, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Yadav, K.K.; Kenney, S.P. Hepatitis E Virus Immunopathogenesis. Pathogens 2021, 10, 1180. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Pierson, F.; Toth, T.; Meng, X. Construction and characterization of infectious cDNA clones of a chicken strain of hepatitis E virus (HEV), avian HEV. J. Gen. Virol. 2005, 86, 2585–2593. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.M.; LeRoith, T.; Pudupakam, R.S.; Pierson, F.W.; Huang, Y.W.; Dryman, B.A.; Meng, X.J. Construction of an infectious cDNA clone of avian hepatitis E virus (avian HEV) recovered from a clinically healthy chicken in the United States and characterization of its pathogenicity in specific-pathogen-free chickens. Vet. Microbiol. 2011, 147, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Debing, Y.; Mishra, N.; Verbeken, E.; Ramaekers, K.; Dallmeier, K.; Neyts, J. A rat model for hepatitis E virus. Dis. Models Mech. 2016, 9, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Yadav, K.K.; Kenney, S.P. Extrahepatic Replication Sites of Hepatitis E Virus (HEV). Zoonotic Dis. 2023, 3, 68–84. [Google Scholar] [CrossRef]
- Anty, R.; Ollier, L.; Péron, J.M.; Nicand, E.; Cannavo, I.; Bongain, A.; Giordanengo, V.; Tran, A. First case report of an acute genotype 3 hepatitis E infected pregnant woman living in South-Eastern France. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2012, 54, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Tabatabai, J.; Wenzel, J.J.; Soboletzki, M.; Flux, C.; Navid, M.H.; Schnitzler, P. First case report of an acute hepatitis E subgenotype 3c infection during pregnancy in Germany. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2014, 61, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Kapur, N.; Thakral, D.; Durgapal, H.; Panda, S.K. Hepatitis E virus enters liver cells through receptor-dependent clathrin-mediated endocytosis. J. Viral Hepat. 2012, 19, 436–448. [Google Scholar] [CrossRef]
- Okamoto, H. Hepatitis E virus cell culture models. Virus Res. 2011, 161, 65–77. [Google Scholar] [CrossRef]
- Shukla, P.; Nguyen, H.T.; Torian, U.; Engle, R.E.; Faulk, K.; Dalton, H.R.; Bendall, R.P.; Keane, F.E.; Purcell, R.H.; Emerson, S.U. Cross-species infections of cultured cells by hepatitis E virus and discovery of an infectious virus-host recombinant. Proc. Natl. Acad. Sci. USA 2011, 108, 2438–2443. [Google Scholar] [CrossRef] [PubMed]
- Sanford, B.J.; Opriessnig, T.; Kenney, S.P.; Dryman, B.A.; Córdoba, L.; Meng, X.J. Assessment of the cross-protective capability of recombinant capsid proteins derived from pig, rat, and avian hepatitis E viruses (HEV) against challenge with a genotype 3 HEV in pigs. Vaccine 2012, 30, 6249–6255. [Google Scholar] [CrossRef] [PubMed]
- Situ, J.; Hon-Yin Lo, K.; Cai, J.P.; Li, Z.; Wu, S.; Hon-Kiu Shun, E.; Foo-Siong Chew, N.; Yiu-Hung Tsoi, J.; Sze-Man Chan, G.; Hei-Man Chan, W.; et al. An immunoassay system to investigate epidemiology of Rocahepevirus ratti (rat hepatitis E virus) infection in humans. JHEP Rep. 2023, 5, 100793. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, K.K.; Hofstetter, J.D.; Kenney, S.P. A Zoonotic Strain of Rocahepevirus ratti Hepatitis E Virus Does Not Replicate Efficiently within Human Placental JEG-3 Cells. Zoonotic Dis. 2024, 4, 123-134. https://doi.org/10.3390/zoonoticdis4020012
Yadav KK, Hofstetter JD, Kenney SP. A Zoonotic Strain of Rocahepevirus ratti Hepatitis E Virus Does Not Replicate Efficiently within Human Placental JEG-3 Cells. Zoonotic Diseases. 2024; 4(2):123-134. https://doi.org/10.3390/zoonoticdis4020012
Chicago/Turabian StyleYadav, Kush Kumar, Jacob D. Hofstetter, and Scott P. Kenney. 2024. "A Zoonotic Strain of Rocahepevirus ratti Hepatitis E Virus Does Not Replicate Efficiently within Human Placental JEG-3 Cells" Zoonotic Diseases 4, no. 2: 123-134. https://doi.org/10.3390/zoonoticdis4020012