Atorvastatin Reduces the Severity of COVID-19: A Nationwide, Total Population-Based, Case-Control Study
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Data Source
2.2. Exposure and Clinical Variables
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Statin Use and COVID-19 Incidence
3.3. Statin Use and COVID-19 Mortality
3.4. Statin Use and COVID-19 Severity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liao, J.K.; Laufs, U. Pleiotropic effects of statins. Annu. Rev. Pharm. Toxicol. 2005, 45, 89–118. [Google Scholar] [CrossRef] [Green Version]
- Puccetti, L.; Pasqui, A.L.; Auteri, A.; Bruni, F. Mechanisms for antiplatelet action of statins. Curr. Drug Targets Cardiovasc. Hematol. Disord. 2005, 5, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Kumar, A.; SenBanerjee, S.; Staniszewski, K.; Parmar, K.; Vaughan, D.E.; Gimbrone, M.A., Jr.; Balasubramanian, V.; García-Cardeña, G.; Jain, M.K. Kruppel-like factor 2 (KLF2) regulates endothelial thrombotic function. Circ. Res. 2005, 96, e48–e57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Wen, X.; Peng, J.; Lu, Y.; Guo, Z.; Lu, J. Systematic review and meta-analysis on the association between outpatient statins use and infectious disease-related mortality. PLoS ONE 2012, 7, e51548. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, E.M.; Restrepo, M.I.; Anzueto, A.; Pugh, J. The effect of prior statin use on 30-day mortality for patients hospitalized with community-acquired pneumonia. Respir. Res. 2005, 6, 82. [Google Scholar] [CrossRef] [Green Version]
- van den Hoek, H.L.; Bos, W.J.; de Boer, A.; van de Garde, E.M. Statins and prevention of infections: Systematic review and meta-analysis of data from large randomised placebo controlled trials. BMJ 2011, 343, d7281. [Google Scholar] [CrossRef] [Green Version]
- Vandermeer, M.L.; Thomas, A.R.; Kamimoto, L.; Reingold, A.; Gershman, K.; Meek, J.; Farley, M.M.; Ryan, P.; Lynfield, R.; Baumbach, J.; et al. Association between use of statins and mortality among patients hospitalized with laboratory-confirmed influenza virus infections: A multistate study. J. Infect. Dis. 2012, 205, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Episcopio, D.; Aminov, S.; Benjamin, S.; Germain, G.; Datan, E.; Landazuri, J.; Lockshin, R.A.; Zakeri, Z. Atorvastatin restricts the ability of influenza virus to generate lipid droplets and severely suppresses the replication of the virus. FASEB J. 2019, 33, 9516–9525. [Google Scholar] [CrossRef]
- Hui, K.P.; Kuok, D.I.; Kang, S.S.; Li, H.S.; Ng, M.M.; Bui, C.H.; Peiris, J.S.; Chan, R.W.; Chan, M.C. Modulation of sterol biosynthesis regulates viral replication and cytokine production in influenza a virus infected human alveolar epithelial cells. Antivir. Res. 2015, 119, 1–7. [Google Scholar] [CrossRef]
- Yuan, S. Statins may decrease the fatality rate of middle east respiratory syndrome infection. mBio 2015, 6, e01120. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Nava, G.; Trelles-Garcia, D.P.; Yanez-Bello, M.A.; Chung, C.W.; Trelles-Garcia, V.P.; Friedman, H.J. Atorvastatin associated with decreased hazard for death in COVID-19 patients admitted to an ICU: A retrospective cohort study. Crit. Care 2020, 24, 429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Qin, J.J.; Cheng, X.; Shen, L.; Zhao, Y.C.; Yuan, Y.; Lei, F.; Chen, M.M.; Yang, H.; Bai, L.; et al. In-hospital use of statins is associated with a reduced risk of mortality among individuals with COVID-19. Cell Metab. 2020, 32, 176–187. [Google Scholar] [CrossRef] [PubMed]
- De Spiegeleer, A.; Bronselaer, A.; Teo, J.T.; Byttebier, G.; De Tré, G.; Belmans, L.; Dobson, R.; Wynendaele, E.; Van De Wiele, C.; Vandaele, F.; et al. The effects of arbs, aceis, and statins on clinical outcomes of COVID-19 infection among nursing home residents. J. Am. Med. Dir. Assoc. 2020, 21, 909–914. [Google Scholar] [CrossRef]
- Lee, C.C.; Lee, M.G.; Hsu, T.C.; Porta, L.; Chang, S.S.; Yo, C.H.; Tsai, K.C.; Lee, M. A population-based cohort study on the drug-specific effect of statins on sepsis outcome. Chest 2018, 153, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Pihl-Jensen, G.; Tsakiri, A.; Frederiksen, J.L. Statin treatment in multiple sclerosis: A systematic review and meta-analysis. CNS Drugs 2015, 29, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Di Bello, E.; Zwergel, C.; Mai, A.; Valente, S. The innovative potential of statins in cancer: New targets for new therapies. Front. Chem. 2020, 8, 516. [Google Scholar] [CrossRef] [PubMed]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic effects of statins on the cardiovascular system. Circ. Res. 2017, 120, 229–243. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Liao, J.K. Pleiotropic effects of statins—Basic research and clinical perspectives. Circ. J. 2010, 74, 818–826. [Google Scholar] [CrossRef] [Green Version]
- Lansberg, P. Pleiotropic effects of statins and beyond. Cardiology 2009, 112, 1–3. [Google Scholar] [CrossRef]
- Mehrbod, P.; Ideris, A.; Omar, A.R.; Hair-Bejo, M. Evaluation of antiviral effect of atorvastatin on H1N1 infection in MDCK cells. Afr. J. Microbiol. Res. 2012, 6, 5715–5719. [Google Scholar] [CrossRef]
- Castiglione, V.; Chiriacò, M.; Emdin, M.; Taddei, S.; Vergaro, G. Statin therapy in COVID-19 infection. Eur. Heart J. Cardiovasc. Pharmacother. 2020, 6, 258–259. [Google Scholar] [CrossRef] [PubMed]
- Sanchis-Gomar, F.; Perez-Quilis, C.; Favaloro, E.J.; Lippi, G. Statins and other drugs: Facing COVID-19 as a vascular disease. Pharmacol. Res. 2020, 159, 105033. [Google Scholar] [CrossRef] [PubMed]
- Fajgenbaum, D.C.; Rader, D.J. Teaching old drugs new tricks: Statins for COVID-19? Cell Metab. 2020, 32, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Milajerdi, A.; Larijani, B.; Esmaillzadeh, A. Statins influence biomarkers of low grade inflammation in apparently healthy people or patients with chronic diseases: A systematic review and meta-analysis of randomized clinical trials. Cytokine 2019, 123, 154752. [Google Scholar] [CrossRef]
- Antonio, V.; Francesco, F. Plausible Positive Effects of Statins in COVID-19 Patient. Cardiovasc. Toxicol. 2021, 21, 781–789. [Google Scholar] [CrossRef]
- Penkauskas, T.; Zentelyte, A.; Ganpule, S.; Valincius, G.; Preta, G. Pleiotropic effects of statins via interaction with the lipid bilayer: A combined approach. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183306. [Google Scholar] [CrossRef]
- Glende, J.; Schwegmann-Wessels, C.; Al-Falah, M.; Pfefferle, S.; Qu, X.; Deng, H.; Drosten, C.; Naim, H.Y.; Herrler, G. Importance of cholesterol-rich membrane microdomains in the interaction of the s protein of sars-coronavirus with the cellular receptor angiotensin-converting enzyme 2. Virology 2008, 381, 215–221. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; Deng, Y.; Guo, X.; Shang, J.; Zhu, D.; Liu, H. Atorvastatin attenuates myocardial remodeling induced by chronic intermittent hypoxia in rats: Partly involvement of TLR-4/MYD88 pathway. Biochem. Biophys. Res. Commun. 2014, 446, 292–297. [Google Scholar] [CrossRef]
- Crunkhorn, S. Statin therapy improves endothelial dysfunction. Nat. Rev. Drug Discov. 2020, 19, 588. [Google Scholar] [CrossRef]
- Masadeh, M.; Mhaidat, N.; Alzoubi, K.; Al-Azzam, S.; Alnasser, Z. Antibacterial activity of statins: A comparative study of atorvastatin, simvastatin, and rosuvastatin. Ann. Clin. Microbiol. Antimicrob. 2012, 11, 13. [Google Scholar] [CrossRef] [Green Version]
- Francesco, F.; Antonio, V. The advantages of drug treatment with statins in patients with SARS-CoV-2 infection. Wien. Klin. Wochenschr. 2021, 133, 958–965. [Google Scholar] [CrossRef]



| Case Subjects | Matched Control Subjects | p * | |||
|---|---|---|---|---|---|
| N (%) | (n = 7723) | (n = 46,231) | |||
| Age, mean (SD) | 55.9 (14.4) | 52.1 (16.2) | − | ||
| Male subjects | 3056 | (39.6) | 21,366 | (46.2) | − |
| City of residence | − | ||||
| Seoul | 515 | (6.7) | 5150 | (11.1) | |
| Daegu | 5036 | (65.2) | 20,108 | (43.5) | |
| Gyung-gi | 435 | (5.6) | 4350 | (9.4) | |
| Gyung-buk | 933 | (12.1) | 8583 | (18.6) | |
| Other | 804 | (10.4) | 8040 | (17.4) | |
| Income | <0.001 | ||||
| Low | 2767 | (35.8) | 12,163 | (26.3) | |
| Middle | 2180 | (28.2) | 14,736 | (31.9) | |
| High | 2776 | (35.9) | 19,332 | (41.8) | |
| Medical history | |||||
| Hypertension | 1914 | (24.8) | 13,991 | (30.3) | <0.001 |
| Diabetes mellitus | 845 | (10.9) | 6152 | (13.3) | <0.001 |
| Dyslipidemia | 1834 | (23.7) | 12,571 | (27.2) | <0.001 |
| Cardiovascular disease | 1468 | (19.0) | 10,822 | (23.4) | <0.001 |
| Ischemic heart disease | 74 | (1.0) | 1339 | (2.9) | <0.001 |
| Peripheral arterial disease | 1253 | (16.2) | 8667 | (18.7) | <0.001 |
| Stroke | 125 | (1.6) | 1353 | (2.9) | <0.001 |
| Heart failure | 207 | (2.7) | 2213 | (4.8) | <0.001 |
| Chronic kidney disease | 62 | (0.8) | 1505 | (3.3) | <0.001 |
| Cancer | 660 | (8.5) | 9148 | (19.8) | <0.001 |
| Medications prescribed | |||||
| Statins | 1162 | (15.0) | 8503 | (18.4) | <0.001 |
| Other lipid lowering agents | 98 | (1.3) | 565 | (1.2) | 0.504 |
| Antiplatelet agents | 682 | (8.8) | 5562 | (12.0) | <0.001 |
| Anticoagulants | 74 | (1.0) | 1135 | (2.5) | <0.001 |
| Case Subjects (n = 7723) | Matched Control Subjects (n = 46,231) | Odds Ratio for COVID-19 (95% Confidence Interval) | ||||
|---|---|---|---|---|---|---|
| N (%) | N (%) | Unadjusted | p | Adjusted * | p | |
| Use of any statins | 1162 (15.0) | 8503 (18.4) | 0.72 (0.67–0.78) | <0.001 | 0.94 (0.85–1.04) | 0.209 |
| Type of statins | ||||||
| Atorvastatin | 550 (7.1) | 4247 (9.2) | 0.71 (0.64–0.78) | <0.001 | 0.92 (0.82–1.05) | 0.210 |
| Rosuvastatin | 470 (6.1) | 504 (1.1) | 0.81 (0.73–0.89) | <0.001 | 0.92 (0.82–1.05) | 0.215 |
| Others | 181 (2.3) | 1198 (2.6) | 0.89 (0.72–1.10) | 0.294 | 1.07 (0.86–1.34) | 0.558 |
| Death (n = 255) | Survival (n = 7468) | Odds Ratio for Death (95% Confidence Interval) | ||||
|---|---|---|---|---|---|---|
| N (%) | N (%) | Unadjusted | p | Adjusted * | p | |
| Use of any statins | 96 (37.7) | 1066 (14.3) | 3.63 (2.79–4.71) | <0.001 | 0.68 (0.45–1.03) | 0.069 |
| Type of statins | ||||||
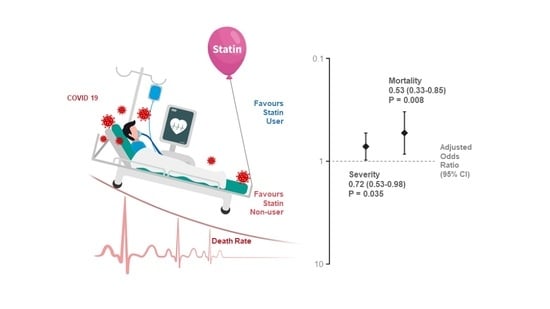
| Atorvastatin | 51 (20.0) | 499 (6.7) | 3.49 (2.54–4.81) | <0.001 | 0.53 (0.33–0.85) | 0.008 |
| Rosuvastatin | 30 (11.8) | 440 (5.9) | 2.13 (1.44–3.16) | <0.001 | 0.80 (0.48–1.33) | 0.391 |
| Others | 18 (7.1) | 163 (2.2) | 4.31 (0.81–2.38) | <0.001 | 1.58 (0.75–3.30) | 0.227 |
| Severe (n = 493) | Mild (n = 7230) | Odds Ratio for Severity (95% Confidence Interval) | ||||
|---|---|---|---|---|---|---|
| N (%) | N (%) | Unadjusted | p | Adjusted * | p | |
| Use of any statins | 158 (32.0) | 1004 (13.9) | 2.93 (2.39–3.57) | <0.001 | 0.72 (0.53–0.98) | 0.035 |
| Type of statins | ||||||
| Atorvastatin | 84 (17.0) | 466 (6.4) | 2.98 (2.32–3.84) | <0.001 | 0.65 (0.46–0.93) | 0.019 |
| Rosuvastatin | 52 (10.5) | 418 (5.8) | 1.92 (1.42–2.60) | <0.001 | 0.74 (0.51–1.09) | 0.129 |
| Others | 28 (5.7) | 153 (2.1) | 2.90 (1.71–4.91) | <0.001 | 1.08 (0.59–1.97) | 0.800 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, D.-H.; Choi, J.; Gwon, J.G. Atorvastatin Reduces the Severity of COVID-19: A Nationwide, Total Population-Based, Case-Control Study. COVID 2022, 2, 398-406. https://doi.org/10.3390/covid2030028
Cho D-H, Choi J, Gwon JG. Atorvastatin Reduces the Severity of COVID-19: A Nationwide, Total Population-Based, Case-Control Study. COVID. 2022; 2(3):398-406. https://doi.org/10.3390/covid2030028
Chicago/Turabian StyleCho, Dong-Hyuk, Jimi Choi, and Jun Gyo Gwon. 2022. "Atorvastatin Reduces the Severity of COVID-19: A Nationwide, Total Population-Based, Case-Control Study" COVID 2, no. 3: 398-406. https://doi.org/10.3390/covid2030028