Phosphorus Recycling, Biocontrol, and Growth Promotion Capabilities of Soil Bacterial Isolates from Mexican Oak Forests: An Alternative to Reduce the Use of Agrochemicals in Maize Cultivation
Abstract
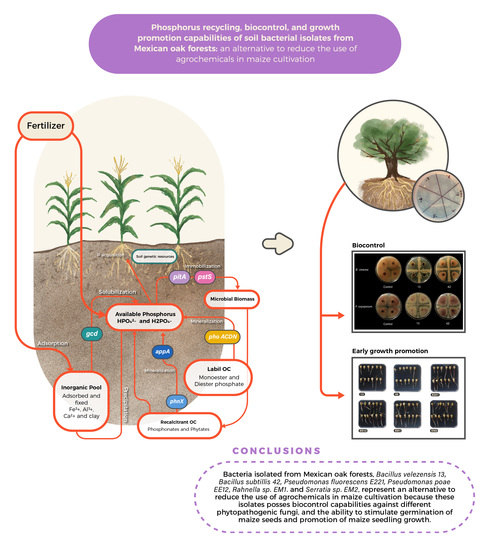
:1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Siderophores Production and Phosphate Solubilization
2.3. Antifungal Activity
2.4. Molecular Characterization of Bacterial Isolates
2.5. Growth Test in Different Phosphorus Sources
2.6. Indole Detection
2.7. Maize Seed Germination Test
2.8. Early Growth Promotion Test in Seedbeds
2.9. Statistical Analysis
3. Results
3.1. Selection and Molecular Characterization of the Bacterial Isolates
3.2. Siderophores Production and Phosphate Solubilization
3.3. Biocontrol Capacity
3.4. Phosphorus Growth Test
3.5. Indole Detection
3.6. Seed Germination
3.7. Early Growth Promotion Test in Seedbeds
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guignard, M.S.; Leitch, A.R.; Acquisti, C.; Eizaguirre, C.; Elser, J.J.; Hessen, D.O.; Leitch, I.J. Impacts of nitrogen and phosphorus: From genomes to natural ecosystems and agriculture. Front. Ecol. Evol. 2017, 5, 70. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.X.; Liu, Y.M.; Zhao, Q.Y.; Cao, W.Q.; Chen, X.P.; Zou, C.Q. Health risk assessment associated with heavy metal accumulation in wheat after long-term phosphorus fertilizer application. Environ. Pollut. 2020, 262, 114348. [Google Scholar] [CrossRef] [PubMed]
- Morée, A.L.; Beusen, A.H.W.; Bouwman, A.F.; Willems, W.J. Exploring global nitrogen and phosphorus flows in urban wastes during the twentieth century. Global Biogeochem. Cycles 2013, 27, 836–846. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Xu, C.C.; Ridoutt, B.G.; Wang, X.C.; Ren, P.A. Nitrogen and phosphorus losses and eutrophication potential associated with fertilizer application to cropland in China. J. Clean. Prod. 2017, 159, 171–179. [Google Scholar] [CrossRef]
- Fischer, P.; Pöthig, R.; Gücker, B.; Venohr, M. Phosphorus saturation and superficial fertilizer application as key parameters to assess the risk of diffuse phosphorus losses from agricultural soils in Brazil. Sci. Total Environ. 2018, 630, 1515–1527. [Google Scholar] [CrossRef] [PubMed]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen–phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, NJ, USA, 2002; pp. 123–138. [Google Scholar]
- Elser, J.; Haygarth, P. Phosphorus Past and Future; Oxford University Press: New York, NY, USA, 2020; pp. 56–62, ISBN/EAN: 9780199916917. [Google Scholar]
- Cordell, D.; White, S. Peak phosphorus: Clarifying the key issues of a vigorous debate about long-term phosphorus security. Sustainability 2011, 3, 2027–2049. [Google Scholar] [CrossRef] [Green Version]
- Schröder, J.J.; Smit, A.L.; Cordell, D.; Rosemarin, A. Improved phosphorus use efficiency in agriculture: A key requirement for its sustainable use. Chemosphere 2011, 84, 822–831. [Google Scholar] [CrossRef]
- Mohammadi, K.; Sohrabi, Y. Bacterial biofertilizers for sustainable crop production: A review. ARPN J. Agric. Biol. Sci. 2012, 7, 307–316. [Google Scholar]
- Restrepo-Franco, G.M.; Marulanda-Moreno, S.; de la Fe-Pérez, Y.; Díaz-de la Osa, A.; Lucia-Baldani, V.; Hernández-Rodríguez, A. Bacterias solubilizadoras de fosfato y sus potencialidades de uso en la promoción del crecimiento de cultivos de importancia económica. Rev. CENIC Cienc. Biol. 2015, 46, 63–76. [Google Scholar]
- Tapia-Torres, Y.; Rodríguez-Torres, M.D.; Elser, J.J.; Islas, A.; Souza, V.; García-Oliva, F.; Olmedo-Álvarez, G. How to live with phosphorus scarcity in soil and sediment: Lessons from bacteria. Appl. Environ. Microbiol. 2016, 82, 4652–4662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perroni, Y.; García-Oliva, F.; Tapia-Torres, Y.; Souza, V. Relationship between soil P fractions and microbial biomass in an oligotrophic grassland-desert scrub system. Ecol. Res. 2014, 29, 463–472. [Google Scholar] [CrossRef]
- Ruttenberg, K.C. The global phosphorus cycle. Treatise Geochem. 2003, 8, 585–643. [Google Scholar]
- Bergkemper, F.; Schöler, A.; Engel, M.; Lang, F.; Krüger, J.; Schloter, M.; Schulz, S. Phosphorus depletion in forest soils shapes bacterial communities towards phosphorus recycling systems. Environ. Microbiol. 2016, 18, 1988–2000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Kooner, R.; Arora, R. Insect pests and crop losses. In Breeding Insect Resistant Crops for Sustainable Agriculture; Springer: Singapore, 2017; pp. 45–66. [Google Scholar] [CrossRef]
- Ab Rahman, S.F.S.; Singh, E.; Pieterse, C.M.; Schenk, P.M. Emerging microbial biocontrol strategies for plant pathogens. Plant Sci. 2018, 267, 102–111. [Google Scholar] [CrossRef] [Green Version]
- Deutsch, C.A.; Tewksbury, J.J.; Tigchelaar, M.; Battisti, D.S.; Merrill, S.C.; Huey, R.B.; Naylor, R.L. Increase in crop losses to insect pests in a warming climate. Science 2018, 361, 916–919. [Google Scholar] [CrossRef] [Green Version]
- INEGI. 2014. Available online: https://www.inegi.org.mx/contenidos/programas/ena/2014/doc/minimonografia/mininacena14.pdf (accessed on 17 May 2021).
- Zepeda-Jazo, I. Manejo sustentable de plagas agrícolas en México. Agric. Soc. Y Desarro. 2018, 15, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Desjardins, A.E. Gibberella from A (venaceae) to Z (eae). Annu. Rev. Phytopathol. 2003, 41, 177–198. [Google Scholar] [CrossRef]
- Roncero, M.I.G.; Hera, C.; Ruiz-Rubio, M.; Maceira, F.I.G.; Madrid, M.P.; Caracuel, Z.; Di Pietro, A. Fusarium as a model for studying virulence in soilborne plant pathogens. Physiol. Mol. Plant Pathol. 2003, 62, 87–98. [Google Scholar] [CrossRef]
- Goswami, R.S.; Kistler, H.C. Heading for disaster: Fusarium graminearum on cereal crops. Mol. Plant Pathol. 2004, 5, 515–525. [Google Scholar] [CrossRef]
- Pegg, G.F.; Tjamos, E.C.; Beckman, C. Pathogenesis in vascular diseases of plants. In Vascular Wilt Diseases of Plants; Springer: Berlin/Heidelberg, Germany, 1989; pp. 51–94. [Google Scholar]
- Michielse, C.B.; Rep, M. Pathogen profile update: Fusarium oxysporum. Mol. Plant Pathol. 2009, 10, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Williamson, B.; Tudzynski, B.; Tudzynski, P.; Van Kan, J.A. Botrytis cinerea: The cause of grey mould disease. Mol. Plant Pathol. 2007, 8, 561–580. [Google Scholar] [CrossRef] [PubMed]
- Wezel, A.; Bellon, S.; Doré, T.; Francis, C.; Vallod, D.; David, C. Agroecology as a science, a movement and a practice. A review. Agron. Sustain. Dev. 2009, 29, 503–515. [Google Scholar] [CrossRef] [Green Version]
- Wezel, A.; Casagrande, M.; Celette, F.; Vian, J.F.; Ferrer, A.; Peigné, J. Agroecological practices for sustainable agriculture. A review. Agron. Sustain. Dev. 2014, 34, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Ryu, C.M.; Farag, M.A.; Hu, C.H.; Reddy, M.S.; Wei, H.X.; Paré, P.W.; Kloepper, J.W. Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 4927–4932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spaepen, S.; Versées, W.; Gocke, D.; Pohl, M.; Steyaert, J.; Vanderleyden, J. Characterization of phenylpyruvate decarboxylase, involved in auxin production of Azospirillum brasilense. J. Bacteriol. 2007, 189, 7626–7633. [Google Scholar] [CrossRef] [Green Version]
- Gaba, V.P. Plant growth regulators in plant tissue culture and development. In Plant Development and Biotechnology; CRC Press: Boca Raton, FL, USA, 2005; pp. 87–99. [Google Scholar]
- Teale, W.D.; Paponov, I.A.; Palme, K. Auxin in action: Signalling, transport and the control of plant growth and development. Nat. Rev. Mol. Cell Biol. 2006, 7, 847–859. [Google Scholar] [CrossRef]
- Möller, B.; Weijers, D. Auxin control of embryo patterning. Cold Spring Harb. Perspect. Biol. 2009, 1, a001545. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Tian, L.; Yang, J.; Zhao, Y.N.; Zhu, Y.X.; Dai, X.; Yang, Z.N. Auxin production in diploid microsporocytes is necessary and sufficient for early stages of pollen development. PLoS Genet. 2018, 14, e1007397. [Google Scholar] [CrossRef] [Green Version]
- Lv, B.; Yan, Z.; Tian, H.; Zhang, X.; Ding, Z. Local auxin biosynthesis mediates plant growth and development. Trends Plant Sci. 2019, 24, 6–9. [Google Scholar] [CrossRef]
- Spaepen, S.; Vanderleyden, J. Auxin and plant-microbe interactions. Cold Spring Harb. Perspect. Biol. 2011, 3, a001438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vega-Celedón, P.; Canchignia Martínez, H.; González, M.; Seeger, M. Biosynthesis of indole-3-acetic acid and plant growth promoting by bacteria. Cultiv. Trop. 2016, 37, 33–39. [Google Scholar]
- Compant, S.; Duffy, B.; Nowak, J.; Clément, C.; Barka, E.A. Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef]
- Calvo, J.; Calvente, V.; de Orellano, M.E.; Benuzzi, D.; de Tosetti, M.I.S. Biological control of postharvest spoilage caused by Penicillium expansum and Botrytis cinerea in apple by using the bacterium Rahnella aquatilis. Int. J. Food Microbiol. 2007, 113, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Hipp, A.L.; Manos, P.S.; González-Rodríguez, A.; Hahn, M.; Kaproth, M.; McVay, J.D.; Cavender-Bares, J. Sympatric parallel diversification of major oak clades in the Americas and the origins of Mexican species diversity. New Phytol. 2018, 217, 439–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deepa, C.K.; Dastager, S.G.; Pandey, A. Isolation and characterization of plant growth promoting bacteria from non-rhizospheric soil and their effect on cowpea (Vigna unguiculata (L.) Walp.) seedling growth. World J. Microbiol. Biotechnol. 2010, 26, 1233–1240. [Google Scholar] [CrossRef]
- Sukweenadhi, J.; Kim, Y.J.; Lee, K.J.; Koh, S.C.; Hoang, V.A.; Nguyen, N.L.; Yang, D.C. Paenibacillus yonginensis sp. nov., a potential plant growth promoting bacterium isolated from humus soil of Yongin forest. Antonie Van Leeuwenhoek 2014, 106, 935–945. [Google Scholar] [CrossRef]
- Pandey, C.; Dheeman, S.; Negi, Y.K.; Maheshwari, D.K. Differential response of native Bacillus spp. isolates from agricultural and forest soils in growth promotion of Amaranthus hypochondriacus. Biotechnol. Res. 2018, 4, 54–61. [Google Scholar]
- Schwyn, B.; Neilands, J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Hernández-León, R.; Rojas-Solís, D.; Contreras-Pérez, M.; del Carmen Orozco-Mosqueda, M.; Macías-Rodríguez, L.I.; Reyes-de la Cruz, H.; Santoyo, G. Characterization of the antifungal and plant growth-promoting effects of diffusible and volatile organic compounds produced by Pseudomonas fluorescens strains. Biol. Control 2015, 81, 83–92. [Google Scholar] [CrossRef]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro, L.; Dunoyer, P.; Jay, F.; Arnold, B.; Dharmasiri, N.; Estelle, M.; Jones, J.D. A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science 2006, 312, 436–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, S.A.; Weber, R.P. Colorimetric estimation of indoleacetic acid. Plant Physiol. 1951, 26, 192–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghiyasi, M.; Zardoshty, M.R.; Mogadam, A.F.; Tajbakhsh, M.; Amirnia, R. Effect of osmopriming on germination and seedling growth of corn (Zea mays L.) seeds. Res. J. Biol. Sci. 2008, 3, 779–782. [Google Scholar]
- Rasband, W.S. ImageJ; US National Institutes of Health: Bethesda, MD, USA, 1997. Available online: http://imagej.nih.gov/ij (accessed on 30 January 2021).
- Jones, B.; Sall, J. JMP statistical discovery software. In Computational Statistics; Wiley Interdisciplinary Reviews: Hoboken, NJ, USA, 2011; Volume 3, pp. 188–194. [Google Scholar]
- Whitelaw, M.A. Growth promotion of plants inoculated with phosphate-solubilizing fungi. Adv. Agron. 1999, 69, 99–151. [Google Scholar]
- Dao, T.H. Extracellular enzymes in sensing environmental nutrients and ecosystem changes: Ligand mediation in organic phosphorus cycling. In Soil Enzymology; Springer: Berlin/Heidelberg, Germany, 2010; Volume 22, pp. 75–102. [Google Scholar] [CrossRef]
- Huang, J.; Su, Z.; Xu, Y. The evolution of microbial phosphonates degradative pathways. J. Mol. Evol. 2005, 61, 682–690. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Debaeke, P.; Steinberg, C.; You, M.P.; Barbetti, M.J.; Aubertot, J.N. Abiotic and biotic factors affecting crop seed germination and seedling emergence: A conceptual framework. Plant Soil 2018, 432, 1–28. [Google Scholar] [CrossRef]
- Cavaglieri, L.; Orlando, J.R.M.I.; Rodríguez, M.I.; Chulze, S.; Etcheverry, M. Biocontrol of Bacillus subtilis against Fusarium verticillioides in vitro and at the maize root level. Res. Microbiol. 2005, 156, 748–754. [Google Scholar] [CrossRef]
- Yánez-Mendizábal, V.; Viñas, I.; Usall, J.; Torres, R.; Solsona, C.; Abadias, M.; Teixidó, N. Formulation development of the biocontrol agent Bacillus subtilis strain CPA-8 by spray-drying. J. Appl. Microbiol. 2012, 112, 954–965. [Google Scholar] [CrossRef]
- Ju, R.; Zhao, Y.; Li, J.; Jiang, H.; Liu, P.; Yang, T.; Liu, X. Identification and evaluation of a potential biocontrol agent, Bacillus subtilis, against Fusarium sp. in apple seedlings. Ann. Microbiol. 2014, 64, 377–383. [Google Scholar] [CrossRef]
- Lawongsa, P.; Boonkerd, N.; Wongkaew, S.; O’Gara, F.; Teaumroong, N. Molecular and phenotypic characterization of potential plant growth-promoting Pseudomonas from rice and maize rhizospheres. World J. Microbiol. Biotechnol. 2008, 24, 1877–1884. [Google Scholar] [CrossRef]
- Adeniji, M.A.; Babalola, O.O. Indigenous Pseudomonas from maize rhizosphere (Zea mays L.) with strong biocontrol potential for fusariosis management. In SIMB Annual Meeting and Exhibition; North-West University: Mmabatho, South Africa, 2017. [Google Scholar]
- Adeniji, A.A.; Aremu, O.S.; Babalola, O.O. Pseudomonas fulva HARBPS9. 1: Candidate anti-Fusarium agent in South Africa. Eur. J. Plant Pathol. 2020, 157, 767–781. [Google Scholar] [CrossRef]
- Ramette, A.; Frapolli, M.; Fischer-Le Saux, M.; Gruffaz, C.; Meyer, J.M.; Défago, G.; Moënne-Loccoz, Y. Pseudomonas protegens sp. nov., widespread plant-protecting bacteria producing the biocontrol compounds 2,4-diacetylphloroglucinol and pyoluteorin. Syst. Appl. Microbiol. 2011, 34, 180–188. [Google Scholar] [CrossRef]
- Ren, Y.; Yao, M.; Chang, P.; Sun, Y.; Li, R.; Meng, D.; Wang, Y. Isolation and characterization of a Pseudomonas poae JSU-Y1 with patulin degradation ability and biocontrol potential against Penicillium expansum. Toxicon 2021, 195, 1–6. [Google Scholar] [CrossRef]
- Dagher, F.; Nickzad, A.; Zheng, J.; Hoffmann, M.; Déziel, E. Characterization of the biocontrol activity of three bacterial isolates against the phytopathogen Erwinia amylovora. Microbiologyopen 2021, 10, e1202. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R.; Penrose, D.M.; Li, J. A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J. Theor. Biol. 1998, 190, 63–68. [Google Scholar] [CrossRef]
- Brimecombe, M.J.; De Leij, F.A.A.M.; Lynch, J.M. Rhizodeposition and microbial populations. In The Rhizosphere: Biochemistry and Organic Substances at the Soil-Plant Interface; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: London, UK; New York, NY, USA, 2007; pp. 73–109. [Google Scholar]
- Tabatabaei, S.; Ehsanzadeh, P.; Etesami, H.; Alikhani, H.A.; Glick, B.R. Indole-3-acetic acid (IAA) producing Pseudomonas isolates inhibit seed germination and α-amylase activity in durum wheat (Triticum turgidum L.). Span. J. Agric. Res. 2016, 14, 15. [Google Scholar] [CrossRef] [Green Version]
- Ambreetha, S.; Chinnadurai, C.; Marimuthu, P.; Balachandar, D. Plant-associated Bacillus modulates the expression of auxin-responsive genes of rice and modifies the root architecture. Rhizosphere 2018, 5, 57–66. [Google Scholar] [CrossRef]
- Agarwal, P.; Singh, P.C.; Chaudhry, V.; Shirke, P.A.; Chakrabarty, D.; Farooqui, A.; Sane, V.A. PGPR-induced OsASR6 improves plant growth and yield by altering root auxin sensitivity and the xylem structure in transgenic Arabidopsis thaliana. J. Plant Physiol. 2019, 240, 153010. [Google Scholar] [CrossRef]
- Probanza, A.; Lucas, J.A.; Acero, N.; Mañero, F.G. The influence of native rhizobacteria on european alder (Alnus glutinosa (L.) Gaertn.) growth. Plant Soil 1996, 182, 59–66. [Google Scholar] [CrossRef]
- Safari, D.; Jamali, F.; Nooryazdan, H.R.; Bayat, F. Evaluation of ACC deaminase producing ‘Pseudomonas fluorescens’ strains for their effects on seed germination and early growth of wheat under salt stress. Aust. J. Crop Sci. 2018, 12, 413–421. [Google Scholar] [CrossRef]
- Moeinzadeh, A.; Sharif-Zadeh, F.; Ahmadzadeh, M.; Tajabadi, F. Biopriming of Sunflower (‘Helianthus annuus’ L.) Seed with ‘Pseudomonas fluorescens’ for Improvement of Seed Invigoration and Seedling Growth. Aust. J. Crop Sci. 2010, 4, 564–570. [Google Scholar]
- Sun, H.; Kong, L.; Du, H.; Chai, Z.; Gao, J.; Cao, Q. Benefits of Pseudomonas poae s61 on Astragalus mongholicus growth and bioactive compound accumulation under drought stress. J. Plant Interact. 2019, 14, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; DeBolt, S.; Ma, Q.; McDermaid, A.; Wang, C.; Shapiro, N.; Kyrpides, N.C. Improved draft genome sequence of Pseudomonas poae A2-S9, a strain with plant growth-promoting activity. Microbiol. Resour. Announc. 2019, 8, e00275-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.S.; Dutta, S.; Ann, M.; Raaijmakers, J.M.; Park, K. Promotion of plant growth by Pseudomonas fluorescens strain SS101 via novel volatile organic compounds. Biochem. Biophys. Res. Commun. 2015, 461, 361–365. [Google Scholar] [CrossRef]
- Tahir, H.A.; Gu, Q.; Wu, H.; Raza, W.; Hanif, A.; Wu, L.; Gao, X. Plant growth promotion by volatile organic compounds produced by Bacillus subtilis SYST2. Front. Microbiol. 2017, 8, 171. [Google Scholar] [CrossRef] [Green Version]
- Helaly, A.A.; Hassan, S.M.; Craker, L.E.; Mady, E. Effects of growth-promoting bacteria on growth, yield and nutritional value of collard plants. Ann. Agric. Sci. 2020, 65, 77–82. [Google Scholar] [CrossRef]
- Vyas, P.; Gulati, A. Organic acid production in vitro and plant growth promotion in maize under controlled environment by phosphate-solubilizing fluorescent Pseudomonas. BMC Microbiol. 2009, 9, 174. [Google Scholar] [CrossRef] [Green Version]
- Toribio-Jiménez, J.; Rodríguez-Barrera, M.Á.; Hernández-Flores, G.; Ruvacaba-Ledezma, J.C.; Castellanos-Escamilla, M.; Romero-Ramírez, Y. Isolation and screening of bacteria from Zea mays plant growth promoters. Rev. Int. Contam. Ambie. 2017, 33, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Dastager, S.G.; Deepa, C.K.; Pandey, A. Potential plant growth-promoting activity of Serratia nematodiphila NII-0928 on black pepper (Piper nigrum L.). World J. Microbiol. Biotechnol. 2011, 27, 259–265. [Google Scholar] [CrossRef]
- Zaheer, A.; Mirza, B.S.; Mclean, J.E.; Yasmin, S.; Shah, T.M.; Malik, K.A.; Mirza, M.S. Association of plant growth promoting Serratia spp. with the root nodules of chickpea. Res. Microbiol. 2016, 167, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Ludueña, L.M.; Anzuay, M.S.; Angelini, J.G.; Barros, G.; Luna, M.F.; del Pilar Monge, M.; Taurian, T. Role of bacterial pyrroloquinoline quinone in phosphate solubilizing ability and in plant growth promotion on strain Serratia sp. S119. Symbiosis 2017, 72, 31–43. [Google Scholar] [CrossRef]
- Kotoky, R.; Nath, S.; Maheshwari, D.K.; Pandey, P. Cadmium resistant plant growth promoting rhizobacteria Serratia marcescens S2I7 associated with the growth promotion of rice plant. Environ. Sustain. 2019, 2, 135–144. [Google Scholar] [CrossRef]
- Vyas, P.; Joshi, R.; Sharma, K.C.; Rahi, P.; Gulati, A.; Gulati, A. Cold-adapted and rhizosphere-competent strain of Rahnella sp. with broad-spectrum plant growth-promotion potential. J. Microbiol. Biotechnol. 2010, 20, 1724–1734. [Google Scholar]
- He, H.; Ye, Z.; Yang, D.; Yan, J.; Xiao, L.; Zhong, T.; Jing, Y. Characterization of endophytic Rahnella sp. JN6 from Polygonum pubescens and its potential in promoting growth and Cd, Pb, Zn uptake by Brassica napus. Chemosphere 2013, 90, 1960–1965. [Google Scholar] [CrossRef]
- Peng, J.; Wu, D.; Liang, Y.; Li, L.; Guo, Y. Disruption of acdS gene reduces plant growth promotion activity and maize saline stress resistance by Rahnella aquatilis HX2. J. Basic Microbiolol. 2019, 59, 402–411. [Google Scholar] [CrossRef]
- Magallon-Servin, P.; Antoun, H.; Taktek, S.; de-Bashan, L.E. Designing a multi-species inoculant of phosphate rock-solubilizing bacteria compatible with arbuscular mycorrhizae for plant growth promotion in low-P soil amended with PR. Biol. Fertil. Soils 2020, 56, 521–536. [Google Scholar] [CrossRef]
- Dinesh, R.; Anandaraj, M.; Kumar, A.; Bini, Y.K.; Subila, K.P.; Aravind, R. Isolation, characterization, and evaluation of multi-trait plant growth promoting rhizobacteria for their growth promoting and disease suppressing effects on ginger. Microbiol. Res. 2015, 173, 34–43. [Google Scholar] [CrossRef]
- Lucy, M.; Reed, E.; Glick, B.R. Applications of free-living plant growth-promoting rhizobacteria. Antonie Van Leeuwenhoek 2004, 86, 1–25. [Google Scholar] [CrossRef]
- Fernández-González, A.J.; Martínez-Hidalgo, P.; Cobo-Díaz, J.F.; Villadas, P.J.; Martínez-Molina, E.; Toro, N.; Fernández-López, M. The rhizosphere microbiome of burned holm-oak: Potential role of the genus Arthrobacter in the recovery of burned soils. Sci. Rep. 2017, 7, 6008. [Google Scholar] [CrossRef] [PubMed]
- Tashi-Oshnoei, F.; Harighi, B.; Abdollahzadeh, J. Isolation and identification of endophytic bacteria with plant growth promoting and biocontrol potential from oak trees. For. Pathol. 2017, 47, e12360. [Google Scholar] [CrossRef]
- Reis, F.; Pereira, A.J.; Tavares, R.M.; Baptista, P.; Lino-Neto, T. Cork Oak Forests Soil Bacteria: Potential for Sustainable Agroforest Production. Microorganisms 2021, 9, 1973. [Google Scholar] [CrossRef] [PubMed]







| Strains | Molecular Analysis | Biocontrol Analysis | Biochemical Analysis | |
|---|---|---|---|---|
| Identification of Isolates Based on Partial Sequencing of the 16S rRNA Gene | Inhibition of B. cinerea Radial Growth (%) | Inhibition of F. oxysporum Radial Growth (%) | Indole Compounds Production | |
| 13 | Bacillus velezensis | 92.4 | 13.5 | - |
| 42 | Bacillus subtillis | 46.9 | 17.3 | - |
| E221 | Pseudomonas fluorescens | 7.1 | 5.9 | + |
| EE12 | Pseudomonas poae | 8.9 | 14.9 | - |
| EM1 | Rahnella sp. | 12.1 | 6.1 | + |
| EM2 | Serratia sp. | 8.2 | 5.5 | + |
| Strains | Phosphorus Sources Used by PGPBs | |||||
|---|---|---|---|---|---|---|
| AlPO4 | Ca(H2PO4)2H2O | 2AEP | FePO4 | KH2PO4 | -P | |
| Bacillus velezensis 13 | + | + | + | + | + | - |
| Bacillus subtillis 42 | + | + | + | + | + | - |
| Pseudomonas fluorescens E221 | + | + | + | + | + | - |
| Pseudomonas poae EE12 | + | + | - | + | + | - |
| Rahnella sp. EM1 | + | + | + | + | + | - |
| Serratia sp. EM2 | + | + | + | + | + | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-León, R.; González-Rodríguez, A.; Tapia-Torres, Y. Phosphorus Recycling, Biocontrol, and Growth Promotion Capabilities of Soil Bacterial Isolates from Mexican Oak Forests: An Alternative to Reduce the Use of Agrochemicals in Maize Cultivation. Appl. Microbiol. 2022, 2, 965-980. https://doi.org/10.3390/applmicrobiol2040074
Hernández-León R, González-Rodríguez A, Tapia-Torres Y. Phosphorus Recycling, Biocontrol, and Growth Promotion Capabilities of Soil Bacterial Isolates from Mexican Oak Forests: An Alternative to Reduce the Use of Agrochemicals in Maize Cultivation. Applied Microbiology. 2022; 2(4):965-980. https://doi.org/10.3390/applmicrobiol2040074
Chicago/Turabian StyleHernández-León, Rocío, Antonio González-Rodríguez, and Yunuen Tapia-Torres. 2022. "Phosphorus Recycling, Biocontrol, and Growth Promotion Capabilities of Soil Bacterial Isolates from Mexican Oak Forests: An Alternative to Reduce the Use of Agrochemicals in Maize Cultivation" Applied Microbiology 2, no. 4: 965-980. https://doi.org/10.3390/applmicrobiol2040074