1. Introduction
Water is one of the most valuable resources, vital for the survival of humans, animals, and plants, and essential for industrial use, energy generation, transportation, agriculture, and livestock development activities such as aquaculture [
1]. Only 0.29% of the world’s water is available to be enjoyed, present in rivers, lakes, ponds, dams, and streams [
1]. As the populace expands, so does water consumption in various areas (IBGE, 2020). The contamination of aquatic zones by wastewater has been a huge problem to tackle, as the lack of sanitation and treatment has contributed to a great deal of environmental and public health damage. Effluents can contain solid or dissolved waste, including sanitary sewage, oils, grease, and industrial substances that can pose health risks when in the environment [
2]. Disposal can be carried out legally if it follows the rules established by CONAMA (National Environmental Council), which determine physicochemical standards such as determination of temperature, color, turbidity, odors, settleable solids, hardness, and determination of dissolved oxygen.
The characteristics of each industrial effluent depend on the process used in the industry, usually consisting of organic compounds and metals that are often bio-accumulative [
3]. Among the various elements used industrially, chromium is a toxic element found in nature in two different forms, trivalent (Cr
3+) and hexavalent (Cr
6+), each of which has different physicochemical characteristics, including mobility, toxicity, and bioavailability [
4,
5]. In its hexavalent form, it is considered more toxic and carcinogenic and can cause damage to the environment and living beings [
4,
6,
7]. Trivalent chromium is more stable, a nutrient found in some foods, which contributes to glucose metabolism, but in concentrations above 1.0 mg L
−1, it becomes harmful to health, and when left free in the environment, it can oxidize to Cr
6+ [
4,
6,
7,
8]. For this reason, several studies related to the remediation of Cr
3+ disposal and contamination are being carried out. Techniques such as ion exchange, membrane filtration, electrocoagulation, filtration, percolation, phytoremediation, reverse osmosis, and removal by precipitation using alkaline oxides are used to treat wastewater contaminated with Cr
3+ [
7,
9,
10].
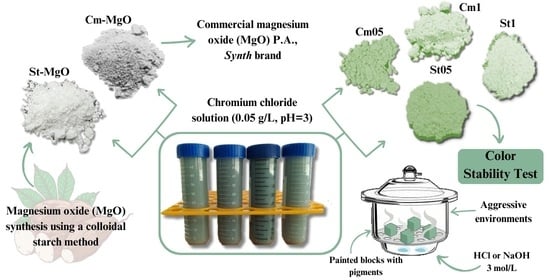
This work aimed to synthesize magnesium oxide (MgO), a single solid with a high ionic character and a crystalline phase known as periclase, stoichiometry, and crystalline structure of a face-centered cubic type and high thermal resistance [
11]. The synthesis was carried out using the colloidal method in a reaction medium with starch extracted from cassava (
Manihot esculenta), a tuber native to Brazil with a high energy value, which can be used as a precursor for synthesis [
12]. Starch is a biodegradable polysaccharide consisting of amylose and amylopectin molecules made up of D-glucose units [
13]. Natural polysaccharides act as low-cost, non-toxic alternative additives produced from plants, acting as complexing agents for central metal ions, and can adapt to the growth of MgO [
14]. Several other studies have shown the advantages of opting for biosynthesis and green fabrication using natural fuels, e.g., Naveed et al. 2023 [
15] in their studies using aqueous extract of Nigella sativa seeds in the synthesis of zero-valent iron nanoparticles (ZVI-NPs) and evaluation of their heavy metal remediation capabilities. Thus, a synthesized oxide and commercial oxide were used as a comparison for the removal of Cr
3+ ions from synthetic wastewater. The removal of chromium by MgO occurs through the coordination of contaminant ions on the surface of the magnesium oxide, resulting in an interaction between the active sites of the oxide and the chromium ions [
16,
17]. After removing the contaminant, the materials obtained a color characteristic of the Cr
3+ ion. They were then characterized and applied as pigments for colorless real estate paint, showing stability of color and structure. The steps involving the methodology for preparing and applying magnesium oxide (MgO) are shown in
Scheme 1.
2. Materials and Methods
2.1. MgO Synthesis and Chromium Removal Tests
The reagents used were of analytical grade: magnesium nitrate (Mg(NO
3)
2∙6H
2O), 98%, Dinâmica); chromium chloride (III) (CrCl
3∙6H
2O, 97%, Sigma Aldrich, Barueri, São Paulo, Brazil); commercial magnesium oxide (MgO, P.A., Synth, Diadema, São Paulo, Brazil). The starch used was extracted directly from the cassava plant (
Manihot esculenta) and used naturally without any chemical treatment. The cassava used was from the region of Palmital City, Paraná. The MgO preparation method involves 3 steps (
Scheme 1):
Step I: The extraction of cassava starch was the initial stage and began by sanitizing the cassava with water and a sponge, followed by extracting all the cassava’s external (brown) peel. The white peel and the inner part of the tuber were then processed in a blender (Britânia brand, model B1000, 1200W power), with a 1:2 proportion of cassava and water (w/w). Following processing, the fiber separated from the juice that was rich in starch. The juice that contained starch was allowed to stand for 24 h, after which the starch was separated from the washing water. Then, using an evaporation dish, the starch was dried in an oven at 60 °C for 30 min, sieved, and stored under refrigeration (−7 °C).
Step II: The methodology for preparing the white matrix was adapted from Primo et al. (2019) [
18]. The preparation of MgO entails the use of 200 g of dry starch and about 800 g of Milli-Q water, obtaining a 1:4 proportion of dry starch and water (m/m). While stirring constantly, 210.9 g of magnesium nitrate was added to the mass of colloidal starch/water emulsion. Following mechanical stirring (600 rpm, 20 min.) at ambient temperature, the suspension was calcined in a muffle furnace at a temperature of 750 °C (for 60 min) and with a heating ramp of 10 °C min
−1. The solid obtained was pulverized and sieved (60 mesh). The synthesized oxide was named St-MgO, while the commercial magnesium oxide used as a comparison was named Cm-MgO.
Step III: The removal tests were carried out simply, using 40 mL of a chromium chloride solution (0.05 g L
−1, pH = 3). The whole process was achieved by adding MgO as a sorbent material and regulating the control conditions (stirring time and centrifugation). The masses of samples used were 500 and 1000 mg. The reaction occurred quickly and had a contact time of 15 min, resulting in constant stirring on a magnetic stirrer. After the contact time had elapsed, the materials were centrifuged, filtered, washed, and dried in an oven at 60 °C for 24 h to obtain the green pigments. The pigments were pulverized in an agate mortar and pestle and sieved (60 mesh) for characterization, and the nomenclature established according to the amount of MgO used is shown in
Table 1.
2.2. Characterizations
The samples were characterized by X-ray diffractometry (XRD) obtained on a Bruker X-ray diffractometer, model D2Phaser, with a copper cathode (λ = 1.5418 Å), potential of 30 kV, current of 10 mA, range between 10° and 80° (2θ), and increment of 0.2°/s. MATCH
® software version 3.16 Build 283 (free-of-charge demo version), with access to the COD-Inorg
® 2020 database was used to index the patterns. Using Bruker’s EVA
® software version 1.1, it was possible to estimate the crystallinity of the samples from the XRD, and the determination of the average crystal size was given by the Scherrer equation (Equation (1)) [
19], where the proportionality constant K was measured from the width of the peak with the greatest intensity at half height, so the minimum value of K = 0.9 and the maximum value K = 1.3 were used.
The elements magnesium and chromium were quantified in an inductively coupled plasma optical emission spectrometer, Thermo Scientific ICP-OES, model iCAP 6500, with iTeVa software, version 1.2.0.30. Samples were dissolved in a solution containing 1.0% of HNO3 (v/v) in Milli-Q water and data were collected in triplicate. The analyzed spectral lines were 279.5 nm and 267.7 nm for magnesium and chromium, respectively. Images of the samples were taken on a TESCAN VEGA3 scanning electron microscope (SEM) at a voltage of 10 kV. The micrographs of the pigments were taken on a FEI Scios dual-beam scanning electron microscope at a voltage of 10 kV.
The nitrogen adsorption–desorption isotherms were obtained using the Quantachrome gas sorption analyzer, model NOVA 2000e, with NovaWin software 11.3. The samples were subjected to vacuum degassing at 200 °C or 250 °C for 2 h and 30 min and the analyses were carried out at liquid nitrogen temperature (−196.15 °C). The specific surface areas of the samples were calculated using the Brunauer–Emmet–Teller (BET) multi-point method. The pore volume and average pore radius were calculated by analyzing the desorption curve using the Barrett–Joyner–Halenda (BJH) model. The samples were characterized using a thermogravimetric analyzer (STA 6000 Perkin Elmer® thermal analyzer), with a temperature sweep from 30 to 1000 °C using a heating rate of 10 °C.min−1, in compressed air (20 mL.min−1), in an alumina crucible. The characterization by FTIR was performed using a Bruker Vertex 70 spectrophotometer using KBr pellets containing around 1% (w/w) of the samples, which were gently mixed and pressed at 10 tons. The spectra were collected in transmission mode by accumulating 32 scans in the region of 400–4000 cm−1, using a resolution of 2 cm−1. Electronic spectra were obtained using Ocean Optics USB-2000 equipment for solid samples, with a tungsten lamp, in the 400–900 nm region, in absorbance mode.
The samples were applied as pigments for commercial residential paint in the incorporation into colorless enamel-type paint, and the ratio of paint to water was 1:2 (
w/
w). The percentage of pigment for incorporation into the paint was 10% for the application of two coats on each plaster block. The colorimetric properties of the pigments in powder and dispersed in the paint were assessed according to the Commission Internationale de I’Eclairage (CIE) using the coordinates L*a*b*C* [
20]. In this system, L* is the degree of clarity or darkness of the color, ranging from white (L* = 100) to black (L* = 0). Positive a* values correspond to the color red, while negative values correspond to green [
20]. Finally, positive b* values correspond to yellow, while negative values correspond to blue. C* values are associated with chroma, which indicates the saturation of the material, and ∆E, which corresponds to color variation (Equation (2)).
2.3. Color Stability Test
The materials were applied as pigments in colorless paints, using Anjo water-based acrylic resin, at a proportion of 10%, and the paint was applied in two coats on square plaster blocks measuring 5 cm on each side. The blocks were exposed to three environments, namely acidic and alkaline, and evaluated by colorimetric analysis at predetermined times. For acid and alkaline media, open Petri dishes containing 50 mL of a solution of hydrochloric acid or sodium hydroxide 3 mol L
−1 were placed in the lower part of a desiccator, and the blocks were added to the upper part, exposed to the vapor of the acid or base. The stability test was adapted from Horsth et al. [
21].
4. Conclusions
The method used to synthesize magnesium oxide from gelatinization with cassava starch proved to be a simple, efficient, low-cost, and reproducible process for obtaining porous oxides.
The X-ray diffractometry (XRD) results identified the formation of single-phase materials (periclase) and crystallinity equal to that of the commercial oxide used as a reference, even when synthesized at relatively low temperatures. This, together with the method of using starch in the reaction medium, led to the formation of magnesium oxide with greater porosity. The SEM images show how the method used can produce different morphologies, such as pseudo-spheres for the synthesized oxide and irregular plates for the commercial oxide. The use of both oxides for the removal of chromium ions had excellent results, with the transformation of the periclase phase to lamellar hydroxides with a hexagonal brucite phase confirmed by FTIR. The data obtained by ICP-OES showed that the samples had values close to the number of magnesium ions before removal, but as the chromium ions were added to the structure, the amount of magnesium decreased, corroborating the ion exchange in the structure. For the SEM data, the morphology obtained was nano-sheets for the green pigments formed from the commercial oxide and rough surface aggregates for the pigments based on the oxide synthesized with starch. The colorimetric properties of the pigments obtained in powder form and applied to colorless paint were analyzed by colorimetry and it was estimated that sample Cm05 was greener than the other pigments (higher negative a* value), both for the powdered samples and when applied to paint. This can be explained by the fact that sample Cm05 had a higher number of chromium ions in mg/g in the structure. After being applied to paint and plaster blocks, the samples were tested for color stability in acidic and basic media, showing little color variation (∆E) after exposure for 240 h.
In terms of the use of chromium (a contaminating heavy metal) as pigments, in an aqueous medium, this pollutant would be more susceptible to mobility, thus affecting a larger contamination area. Depending on the concentration, it would affect the soil near rivers and lakes. When removed from the aqueous medium by MgO nanoparticles, magnesium is substituted by the contaminating chromium ions, due to the ionic proximity between them. Therefore, by removing the harmful Cr3+ ions and trapping them in the structure, the Mg2+ ions are released, which is favorable to the environment. This concluded that it was feasible to prepare magnesium oxide and use it as a material to remove chromium from water as contaminating metal ions and then apply it as a pigment in commercial paint.
As prospects, the work could still encompass other methods to test the stability of chromium ions in the MgO structure, both in powdered pigment and in color stability and photoaging tests. It could also be explored for application as pigments in other dispersion media, such as in ceramic matrices and epoxy resins.