Phytochemical and In Vitro Cytotoxic Screening of Chloroform Extract of Ehretia microphylla Lamk
Abstract
:1. Introduction
2. Results
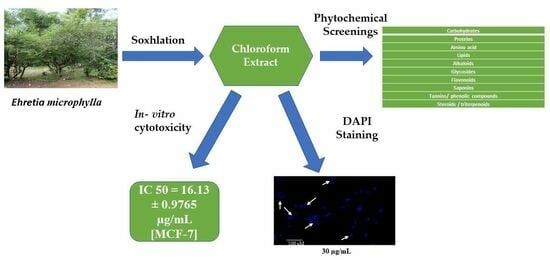
2.1. Plant Extract
2.2. Phytochemical Screening of Chloroform Extract of E. microphylla
2.3. Thin-Layer Chromatography (TLC) Finger Printing
2.4. Biological Evaluation
2.4.1. In Vitro Cytotoxicity
2.4.2. Effect of Chloroform Extract on Nuclear Morphology
3. Discussions
4. Materials and Methods
4.1. Plant Materials
4.2. Chemicals and Reagents
4.3. Preparation of the Extract
4.4. Phytochemical Screening of Extract of E. microphylla
4.5. Thin-Layer Chromatography (TLC) of Extracts
4.6. Cell Culture, Growth Conditions, and Treatment
4.7. Biological Evaluation
4.7.1. In Vitro Cytotoxic Assay
4.7.2. Effect of Chloroform Extract of E. microphylla on Nuclear Morphology
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Horn, L.; Pao, W.; Johnson, D.H. Neoplasms of the lung, Chapter 89. In Harrisons Principles of Internal Medicine, 18th ed.; Longo, D.L., Kasper, D.L., Jamson, J.L., Fauci, A.S., Hauser, S.L., Loscalzo, J., Eds.; MacGraw-Hill: New York, NY, USA, 2012; ISBN 978-0-07-174889-6. [Google Scholar]
- Kumar, D.; Sharma, P.; Singh, H.; Nepali, K.; Gupta, G.K.; Jain, S.K.; Ntie-Kang, F. The value of pyrans as anticancer scaffolds in medicinal chemistry. RSC Adv. 2017, 7, 36977. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Tang, M.; Liu, Y.; Zhang, Z.; Lu, R.; Lu, J. Apigenin inhibits cell proliferation, migration, and invasion by targeting Akt (Protein kinase B) in the A549 human lung cancer cell line. Anticancer Drugs 2017, 28, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Jain, S.K. A Comprehensive Review of N-Heterocycles as Cytotoxic Agents. Curr. Med. Chem. 2016, 23, 4338–4394. [Google Scholar] [CrossRef]
- Singh, S.; Jarial, R.; Kanwar, S.S. Therapeutic effect of herbal medicines on obesity, herbal pancreatic lipase inhibitors. Wudpecker J. Med. Plants. 2013, 2, 53–65. [Google Scholar]
- Kumar, D.; Singh, O.; Nepali, K.; Bedi, P.; Qayum, A.; Singh, S.; Jain, S.K. Naphthoflavones as Antiproliferative Agents: Design, Synthesis and Biological Evaluation. Anticancer Agents Med. Chem. 2016, 16, 881–890. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, R.; Rao, H.S.; Kumar, D. Phytochemistry and Medicinal Attributes of A. Scholaris: A Review. Int. J. Pharm. Sci. Res. 2015, 6, 505–513. [Google Scholar]
- Kumar, D.; Malik, F.; Bedi, P.M.S.; Jain, S. 2,4-diarylpyrano[3,2-c]chromen-5(4H)-ones as coumarin-chalcone conjugates: Design, synthesis and biological evaluation as apoptosis inducing agents. Chem. Pharm. Bull. 2016, 64, 399–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiselova, Y.; Ivanova, D.; Chervenkov, T.; Gerova, D.; Galunska, B.; Yankova, T. Correlation between the in vitro antioxidant activity and polyphenol content of aqueous extracts from Bulgarian herbs. Phytother. Res. 2006, 20, 961–965. [Google Scholar] [CrossRef]
- Kumar, D.; Nepali, K.; Bedi, P.M.S.; Kumar, S.; Malik, F.; Jain, S. 4,6-diaryl Pyrimidones as Constrained Chalcone Analogues: Design, Synthesis and Evaluation as Antiproliferative Agents. Anticancer Agents Med. Chem. 2015, 15, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Singla, R.K.; Sharma, P.; Dubey, A.K.; Gundamaraju, R.; Kumar, D.; Kumar, S.; Madaan, R.; Shri, R.; Tsagkaris, C.; Parisi, S.; et al. Natural Product-Based Studies for the Management of Castration-Resistant Prostate Cancer: Computational to Clinical Studies. Front. Pharmacol. 2021, 12, 732266. [Google Scholar] [CrossRef] [PubMed]
- Cagampan, D.M.; Lacuata, K.A.; Ples, M.B.; Vitor, R.J.S. Effect of Ehretia microphylla on the blood cholesterol and weight of ICR mice (Mus musculus). Natl. J. Physiol. Pharm. Pharmacol. 2018, 8, 983–987. [Google Scholar] [CrossRef]
- Sharma, P.; Shri, R.; Ntie-Kang, F.; Kumar, S. Phytochemical and Ethnopharmacological Perspectives of Ehretia laevis. Molecules 2021, 26, 3489. [Google Scholar] [CrossRef]
- Demetrio, L.V.J.; Jeannie, I.A.; Juliana, J.M.P.; Esperanza, C.C.; Windell, L.R. Antibacterial activities of ethanol extracts of Philippine medicinal plants against multidrug-resistant bacteria. Asian Pac. J. Trop. Biomed. 2015, 5, 532–540. [Google Scholar]
- Murugesa, M.K.S. Gunapadam. Part-I; Tamil Nadu Siddha Medical Board: Chennai, India, 1956; p. 274. [Google Scholar]
- Kaur, R.; Sharma, P.; Gupta, G.K.; Ntie-Kang, F.; Kumar, D. Structure Activity Relationship and Mechanistic Insights for Anti-HIV Natural Products. Molecules 2020, 25, 2070. [Google Scholar] [CrossRef]
- Kumar, D.; Sharma, P.; Kaur, R.; Lobe, M.M.; Gupta, G.K.; Ntie-Kang, F. In search of therapeutic candidates for HIV/AIDS: Rational approaches, design strategies, structure–activity relationship and mechanistic insights. RSC Adv. 2021, 11, 17936–17964. [Google Scholar] [CrossRef]
- Roeder, E.; Wiedenfeld, H. Plants containing pyrrolizidine alkaloids used in the traditional Indian medicine—Including ayurveda. Pharmazie 2013, 68, 83–92. [Google Scholar]
- Vista, F.E.S.; Dalmacio, L.M.M.; Corales, L.G.M.; Salem, G.M.; Galula, J.U.; Chao, D.Y. Antiviral Effect of Crude Aqueous Extracts from Ten Philippine Medicinal Plants against Zika virus. Acta Med. Phillipina 2022, 54, 195–202. [Google Scholar] [CrossRef]
- Bhatia, P.; Sharma, A.; George, A.J.; Anvitha, D.; Kumar, P.; Dwivedi, V.P.; Chandra, N.S. Antibacterial activity of medicinal plants against ESKAPE: An update. Heliyon 2021, 7, e06310. [Google Scholar] [CrossRef]
- Zheng, X.L.; Wei, J.H.; Sun, W.; Li, R.T.; Liu, S.B.; Dai, H.F. Ethnobotanical study on medicinal plants around Limu Mountains of Hainan Island, China. J. Ethnopharmacol. 2013, 148, 964–974. [Google Scholar] [CrossRef]
- Tsaang Gubat or Wild Tea (Ehretia microphylla Lam.). Available online: http://www.philippineherbalmedicine.org/tsaang_gubat.htm (accessed on 14 August 2022).
- Anonymous. The Wealth of India; CSIR: New Delhi, India, 1952; Volume 3, p. 129. [Google Scholar]
- Dapar, M.L.G.; Meve, U.; Liede-Schumann, S.; Alejandro, G.J.D. Ethnomedicinal appraisal and conservation status of medicinal plants among the Manobo tribe of Bayugan City, Philippines. Biodiversitas 2020, 21, 3843–3855. [Google Scholar] [CrossRef]
- Wall, M.E. Camptothecin and taxol: Discovery to clinic. Med. Res. Rev. 1998, 18, 299–314. [Google Scholar] [CrossRef]
- Kaur, T.; Sharma, P.; Gupta, G.; Ntie-Kang, F.; Kumar, D. Treatment of tuberculosis by natural drugs: A review. Plant Arch. 2019, 19, 2168–2176. [Google Scholar]
- Rimando, A.M.; Inoshiri, S.; Otsuka, H.; Kohda, H.; Yamasaki, K.; Padolina, W.G.; Torres, L.; Quintana, E.G.; Cantoria, M.C. Screening for mast cell histamine release inhibitory activity of Philippine medicinal plants. Active constituent of Ehretia microphylla. Jpn. J. Pharmacog. 1987, 41, 242–247. [Google Scholar]
- Cordero, M.; Leon-Gonzalez, A.J.; Montano, J.M.C.; Moron, E.B.; Lazaro, L.M. Pro-oxidant natural products as anticancer agents. Curr. Drug Targets. 2012, 13, 1006–1028. [Google Scholar] [CrossRef]
- Yokoyama, C.; Sueyoshi, Y.; Ema, M.; Mori, Y.; Takaishi, K.; Hisatomi, H. Induction of oxidative stress by anticancer drugs in the presence and absence of cells. Oncol. Lett. 2017, 14, 6066–6070. [Google Scholar] [CrossRef]
- Chopra, R.N.; Nayar, S.L.; Chopra, I.C. Glossary of Indian Medicinal Plants; Council of Scientific and Industrial Research: New Delhi, India, 1986; p. 104. [Google Scholar]
- Safe, S.H.; Prather, P.L.; Brents, L.K.; Chadalapaka, G.; Jutooru, I. Unifying mechanisms of action of the anticancer activities of triterpenoids and synthetic analogs. Anticancer Agents Med. Chem. 2012, 12, 1211–1220. [Google Scholar] [CrossRef]
- Kudera, T.; Fiserova, B.; Korytakova, M.; Doskocil, I.; Salmonova, H.; Tulin, E.E.; Nguon, S.; Bande, M.M.; Kokoska, L. In vitro Selective Antibacterial and Antiproliferative Effects of Ethanolic Extracts from Cambodian and Philippine Plants Used in Folk Medicine for Diarrhea Treatment. Front. Pharmacol. 2021, 12, 746808. [Google Scholar] [CrossRef]
- Stuart, G.U. List of Philippine Herbal Medicinal Plants. Philippine Medicinal Plants. 2017. StuartXchange-SX. Available online: http://www.stuartxchange.org/CompleteList.html (accessed on 14 August 2022).
- Shukla, A.; Kaur, A. A systematic review of traditional uses bioactive phytoconstituents of genus Ehretia. Asian J. Pharmaceut. Clin. Res. 2018, 11, 6–17. [Google Scholar] [CrossRef]
- Simpol, L.R.; Otsuka, H.; Ohtani, K. Nitrile glucosides and rosmarinic acid, the histamine inhibitor from Ehretia philippinensis. Phytochemistry 1994, 36, 91–95. [Google Scholar] [CrossRef]
- Henry, I.A.B.; Alfred, J.H.R.; Kimbberly, A.S.; Allister, M.T.T.; Alicia, I.Y.; Tooba, M.; Jitbanjong, T.; Veeranoot, N. Recommended Medicinal Plants as Source of Natural Products: A Review. Digital Chin. Med. 2018, 1, 131–142. [Google Scholar]
- Legaspi, C.L.B.; Bagaoisan, D.M.A. Ehretia microphylla Tablet Formulation for Biliary and Gastrointestinal Colic: A Review of its Phytochemical Constituents, Pharmacologic Activities and Clinical Researches. Acta Med. Phillipina 2020, 54, 80–85. [Google Scholar] [CrossRef]
- Nayak, J.; Basak, U.C. Antioxidant potential of some lesser known wild edible fruits of Odisha. Eur. J. Exp. Biol. 2015, 5, 60–70. [Google Scholar]
- Ghante, M.H.; Jamkhande, P.G. Role of Pentacyclic Triterpenoids in Chemoprevention and Anticancer Treatment: An Overview on Targets and Underling Mechanisms. J. Pharmacopunct. 2019, 22, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Abdal Dayem, A.; Choi, H.Y.; Yang, G.M.; Kim, K.; Saha, S.K.; Cho, S.G. The Anti-Cancer Effect of Polyphenols against Breast Cancer and Cancer Stem Cells: Molecular Mechanisms. Nutrients 2016, 8, 581. [Google Scholar] [CrossRef]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef] [Green Version]
- Mukharjee, P.K. Quality Control of Herbal Drug, 1st ed.; Business Horizon Publication: New Delhi, India, 2002; pp. 186–193. [Google Scholar]
- Sharma, P.; Kumar, D.; Shri, R.; Kumar, S. Mechanistic insights and docking studies of phytomolecules as potential candidates in the management of cancer. Curr. Pharma. Design. 2022; advance online publication. [Google Scholar] [CrossRef]
- Madaan, R.; Kumar, D.; Singla, R.K.; Kumar, S.; Sharma, P.; Dubey, A.K.; Shen, B. Mechanistic Insight of Pharmacological and nanotechnological perspectives of Bergenin. Curr. Top. Med. Chem. 2022, 22, 132–149. [Google Scholar] [CrossRef]
- WHO. Quality Control Methods for Medicinal Plant Materials; World Health Organization: Geneva, Switzerland, 2010.
- Khandelwal, K.R. Preliminary Phytochemical Screening in Practical Pharmacognosy; Nirali Parkashan: Pune, India, 2004; pp. 1156–1449. [Google Scholar]
- Keepers, Y.P. Comparison of the Sulforhodamine B Protein and Tetrazolium (MTT) Assays for in vitro Chemosensitivity Testing. Eur. J. Cancer 1991, 27, 897–900. [Google Scholar] [CrossRef] [Green Version]
- Skehan, P.; Storeng, R. New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. J. Nat. Cancer Instit. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Akindele, A.J.; Wani, Z.A.; Sharma, S.; Mahajan, G.; Satti, N.K.; Adeyemi, O.O.; Mondhe, D.M.; Saxena, A.K. In vitro and In vivo Anticancer Activity of Root Extracts of Sansevieria liberica Gerome and Labroy (Agavaceae). Evid. Based Complement Alternat. Med. 2015, 15, 560404. [Google Scholar]
- Kumar, A.; Qayum, A.; Sharma, P.R.; Singh, S.K.; Shah, B.A. Synthesis of β-boswellic acid derivatives as cytotoxic and apoptotic agents. Bioorg. Med. Chem. Lett. 2016, 26, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Sharma, P.; Nepali, K.; Mahajan, G.; Mintoo, M.J.; Singh, A.; Singh, G.; Mondhe, D.M.; Singh, G.; Jain, S.K.; et al. Antitumour, acute toxicity and molecular modeling studies of 4-(pyridin-4-yl)-6-(thiophen-2-yl)pyrimidin-2(1H)-one against Ehrlich ascites carcinoma and sarcoma-180. Heliyon 2018, 4, e00661. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, G.; Sharma, P.; Qayum, A.; Mahajan, G.; Mintoo, M.J.; Singh, S.K.; Mondhe, D.M.; Bedi, P.; Jain, S.K.; et al. 4-aryl/heteroaryl-4H-fused Pyrans as Anti-proliferative Agents: Design, Synthesis and Biological Evaluation. Anticancer Agents Med. Chem. 2018, 18, 57–73. [Google Scholar] [CrossRef]
- Mihaljevic, A.L.; Michalski, C.W.; Friess, H.; Kleeff, J. Molecular mechanism of pancreatic cancer-Understanding proliferation, invasion, and metastasis. Langenbeck’s Arch. Surg. 2010, 395, 295–308. [Google Scholar] [CrossRef]


| Sr. No. | Extract (g/Kg) | Percentage Yield | Color | Physical Appearance |
|---|---|---|---|---|
| 1 | Chloroform (54.347) | 5.43 | Green | Sticky mass |
| Sr. No. | Class of Phytoconstituents | Tests | Chloroform |
|---|---|---|---|
| 1 | Carbohydrates | Molisch’s test | − |
| 2 | Proteins | Biuret test | − |
| 3 | Amino acid | Ninhydrin test | − |
| 4 | Lipids | Solubility test | + |
| 5 | Alkaloids | Mayer’s test | − |
| 6 | Glycosides | Legal test | − |
| 7 | Flavonoids | Shinoda test | − |
| 8 | Saponins | Froth test | + |
| 9 | Tannins and phenolic compounds | Ferric chloride test | + |
| 10 | Steroids and triterpenoids | Liebermann–Burchard test | + |
| S. No. | Extract | Solvent System | No. of Spots | Rf Value |
|---|---|---|---|---|
| 1 | Chloroform extract | Hexane: ethyl acetate | 5 | 0.57, 0.65, 0.78, 0.87, 0.92 |
| Entry | HCT-116 (Colon Cancer) | MiaPaCa-2 (Pancreatic Cancer) | PC-3 (Prostate Cancer) | A-549 (Lung Cancer) | HL-60 (Leukemia Cancer) | MCF-7 (Breast Cancer) |
|---|---|---|---|---|---|---|
| PSEM | 75.45 ± 0.0399 | 19.36 ± 0.0422 | 74.50 ± 0.0473 | 77.93 ± 0.0451 | 34.67 ± 0.0410 | 85.55 ± 0.0389 |
| Entry | HCT-116 (Colon Cancer) | MiaPaCa-2 (Pancreatic Cancer) | PC-3 (Prostate Cancer) | A-549 (Lung Cancer) | HL-60 (Leukemia Cancer) | MCF-7 (Breast Cancer) | FR2 (Normal Human Breast Epithelial) |
|---|---|---|---|---|---|---|---|
| PSEM | 26.40 ± 1.2445 | ND | 21.56 ± 1.5473 | 29.47 ± 1.6532 | ND | 16.13 ± 0.9765 | ˃97 |
| Adriamycin | ------ | 1.75 ± 0.143 | ------ | ------ | ------ | 1.10 ± 0.437 | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, P.; Shri, R.; Kumar, S. Phytochemical and In Vitro Cytotoxic Screening of Chloroform Extract of Ehretia microphylla Lamk. Stresses 2022, 2, 384-394. https://doi.org/10.3390/stresses2040027
Sharma P, Shri R, Kumar S. Phytochemical and In Vitro Cytotoxic Screening of Chloroform Extract of Ehretia microphylla Lamk. Stresses. 2022; 2(4):384-394. https://doi.org/10.3390/stresses2040027
Chicago/Turabian StyleSharma, Pooja, Richa Shri, and Suresh Kumar. 2022. "Phytochemical and In Vitro Cytotoxic Screening of Chloroform Extract of Ehretia microphylla Lamk" Stresses 2, no. 4: 384-394. https://doi.org/10.3390/stresses2040027