1. Introduction
Night-time pain is a common problem reported in those with knee-osteoarthritis (knee-OA) [
1]. The prevalence of night-time knee pain has been shown to be associated with the severity of knee-OA [
2]. Indeed, persistent night-time pain is considered as an indication for knee replacement surgery [
3,
4]. Knee pain may become worse at night because levels of the anti-inflammatory hormone cortisol are naturally lower at night. It has also been suggested that staying still in one position, as can occur periodically when lying in bed, might cause joints to become stiff, which can exacerbate knee pain [
5]. Woolhead and colleagues [
6] reported that people suffering knee-OA and/or chronic knee-pain, often place wedged cushioning under the knees in an attempt to alleviate the knee-pain and discomfort they experience when lying in bed. The use of such cushioning changes the resting angle at the knee and/or changes how the limb is supported. This implies that when the limb is in certain resting positions/postures (when not using a cushion), knee structures may be exposed to some level of loading: placing a cushion under the knee attempts to alleviate such loading. The effect of such static loading is the focus of the current study.
Static varus-loading, causing supra-physiological tibiofemoral compression, has been shown, in the rat knee, to produce degenerative changes in medial compartment joint cartilage including a decrease in cartilage modulus (stiffness) and thickness and an increase in matrix loss [
7]. Additionally, in the rabbit knee, fifty minutes of isometric sub-maximal/physiological loading has been shown to lead to the death of cells (chondrocytes) that produce and maintain cartilage matrix [
8]. Chondrocyte death and matrix loss are features that have been shown to be factors in the onset and progression of osteoarthritis [
9,
10]. More generally, the above findings suggest that prolonged static joint loading is a factor in knee-OA. This helps explain why occupations involving doing heavy work/lifting while standing or simply involving prolonged periods of standing have been associated with a higher incidence of having knee-OA (adjusted odds ratio of 1.2 CI 1.02–1.46 [
11], or an odds ratio of 1.4–2.1, which compares to 0.7 for those occupations involving sitting 50% of the time [
12]).
In vivo studies in humans, determining the effects of static loading on knee cartilage, have used imaging techniques to investigate how cartilage responds to loading [
13]. Medial meniscal extrusion (MME) is a measure of how far the medical meniscus protrudes from the medial edge of the tibiofemoral joint line [
13]. The menisci distribute load and reduce friction and serve as shock absorbers. Menisci degeneration (or injury) can result in meniscus extrusion. Although MME has traditionally been measured using MRI in unloaded knees (patient supine), recent use of ultrasound techniques allows MME to be measured for when the knee is loaded during standing [
14]. In comparison to healthy asymptomatic individuals, MME has been shown to be greater in those with knee-OA for both standing and supine positions [
15], which highlights that those with knee-OA typically have meniscus pathology/degradation [
16]. As such, and not surprisingly, MME correlates strongly with tibiofemoral cartilage loss and is an independent predictor of knee-OA progression [
16,
17]. Once knee-OA progresses, the cartilage degradation is rarely reversed, and for those with severe degradation, this can mean that their physical functioning is compromised and they can experience chronic pain including night-time knee pain [
18].
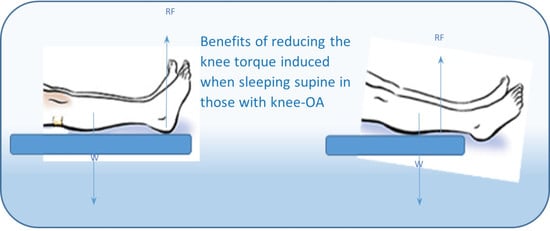
Work recently completed in our laboratory has shown that when a person is lying supine, an external knee extension joint torque is induced in the knee, and the magnitude of this induced torque is comparable to that for standing ([
19], see
Figure 1). Because the heel protrudes “posteriorly” relative to the distal end of the leg, the knee extension torque experienced when lying supine is a consequence of the contact/reaction forces from the support surface (reaction to the weight of the leg) acting predominantly through the heel. Note, in this previous study, participants laid on a solid surface not a mattress. When lying supine on a cushioned mattress, the calves would also contact the mattress, which would reduce the contact forces acting through the heel and thus reduce the induced knee extension toque. Thus, the knee torque data presented in our previous study [
19] (
Figure 1) represents a worst-case scenario. Use of a very firm mattress, for example, certain types of orthopaedic mattresses, may approach this worst-case scenario. In addition, previous research has shown that, when lying in a supine position, the contact pressures at the heel are consistently higher than other locations in the lower extremity [
20,
21,
22]. Due to having a relatively small contact area, it would be expected that heel pressures would be higher than other locations of the lower leg. However, such research indicates that the heel is consistently a site where contact forces from the mattress act. These heel contact forces are likely to vary according to mattress firmness, as more or less weight of the lower leg is supported through the calf. However, any amount of force acting through the heel will tend to create a knee extension torque, since such forces have a relatively long moment arm about the knee. With reaction forces acting at the heel when lying supine, the external joint torque induced “pushes” the knee into full extension. Having the knee pushed into/held in full extension may cause partial loading of internal structures, and as people spend many hours in bed, such partial loading may be experienced for prolonged periods in those who regularly sleep in a supine position.
As highlighted above, people suffering knee-OA and/or chronic knee pain often place wedged cushioning under the knees in an attempt to alleviate the knee pain and discomfort they experience when lying in bed [
6]. Placing a cushion under the knee keeps the joint slightly flexed, which prevents the knee being held in full extension when lying supine, and thus reduces the chances of partially loading internal knee structures. This may explain why the use of a cushion can help alleviate the knee pain and discomfort. However, it was reported in the same study that many found the presence of a cushion awkward when having to change position in bed, and therefore, their use was often more of a hindrance than a help [
6]. An alternative approach for preventing the knee being “pushed” into full extension when lying supine would be to change sleeping position so that the feet hang over the end of the mattress. This position eradicates having the contact/reaction forces acting at the heel, so instead the weight of the lower limb will be supported through the calf, which will reduce the induced knee torque. The work completed in our laboratory demonstrated that, when lying supine with the feet hanging over the end of the support surface, the induced knee torque was reduced to near zero ([
19], see
Figure 1). Note, when lying supine with feet over the end of the support, the line of action of the reaction force from the mattress (RF
vert) acts closer to the line of action of the segment’s weight (W
seg), which is why the induced knee-extension torque reduces to near zero. This indicates that, for those with knee-OA who routinely spend prolonged periods sleeping on their backs, sleeping with the feet hanging over the end of the mattress could prevent them from experiencing prolonged periods with the knee held in full extension. This suggests that, for those with knee-OA who routinely sleep on their backs, the “intervention” of switching to lying with feet over the end of the mattress may reduce the pain and associated physical problems they suffer. The aim of this pilot trial therefore was to evaluate, in patients with knee-OA who routinely sleep on their backs, whether the intervention of changing sleeping position so the feet hang over the end of the mattress would ameliorate the knee pain and associated physical problems they suffer.
2. Materials and Methods
2.1. Study Design and Setting
We conducted a small-scale (preliminary) randomised trial (
ClinicalTrials.gov registration No. NCT04188054; retrospectively registered) to evaluate if the intervention of changing sleeping position so that the feet hang over the end of the mattress resulted in improvements in knee pain and associated physical problems in knee-OA sufferers who customarily sleep supine. The trial was conducted in accordance with CONSORT 2010 guidelines [
24]; see CONSORT 2010 Checklist (
Online Resource S1).
The study was undertaken within a General Practitioners (GPs) Centre in northern England (Idle Medical Centre, Bradford, West Yorkshire), from where all the study’s participants were recruited.
2.2. Recruitment and Eligibility
Posters/leaflets were displayed in the Centre, highlighting that individuals with knee-OA and/or chronic knee pain willing to take part in a research study about chronic knee pain should make an appointment to see a particular GP at the centre (Doctor Bhattacharjee, who is one of the authors of the current paper (author CB)). Dedicated “research” slots outside normal consultation times were used to inform patients of the nature of the study and what involvement in it would mean. The average time spent with each patient was around 20 min.
Those with chronic knee pain were sent for X-ray as part of their usual care. All volunteer patients (n = 70) with confirmed tibiofemoral-OA and who met the inclusion/exclusion criteria were invited to take part. The principal inclusion criteria were (i) the presence of mild-to-moderate or severe tibiofemoral-OA (either or both knees): the severity of OA was reported by a consultant radiologists and based on their X-rays taken of each patient; and (ii) a self-reported indication from the patient that they routinely spend extended periods lying on their back when sleeping. The exclusion criteria were as follows: those with: chronic cardiopulmonary problems; lower-limb joint (or joint surface) replacement and/or previous knee surgery within previous 12 months; unable to lie supine in bed (medical or practical reason); diagnosed with inflammatory arthropathy (e.g., rheumatoid arthritis); peripheral neuropathy or other sensory problems; steroid injection in the knee within previous 3 months; under 18 years of age; unable to give consent due to lack of mental capacity; currently involved in any research study related to knee-OA. The tenets of the Declaration of Helsinki were observed, and all participants provided written informed consent to partake in the study, which was obtained by Doctor Bhattacharjee during a follow-up GP appointment.
Recruitment occurred during the period between 19 March 2018 and 10 December 2018. From those eligible (57/70), 95% (54/57) initially agreed to participate in the research, from which 94% consented to participate (51/54): 33% were male and 67% were females.
2.3. Accounting for All Participants
The study flow diagram of participant recruitment and retention is presented in
Figure 2. Impending hospital appointments for total knee replacement and anticoagulant treatment were the main reason for ineligibility. Of the 51 participants who started the study, two control participants left part-way through without giving a reason, and then seven more participants were lost to follow-up for various reasons. Only the data for the remaining participants (n = 42) were included in the statistical analysis. The characteristics of the participants are presented in
Table 1, divided into the intervention and control groups. There were no significant differences between groups in any of the participant characteristics (
p > 0.3).
2.4. Outcome Measures
A recent systematic review highlighted that the Knee injury and Osteoarthritis Outcome Score questionnaire (KOOS, [
25]) has good internal consistency, test-retest reliability and construct validity, and hence it is a valid measure to use to evaluate patients with different knee conditions and to use in both the young and old [
26]. The primary outcome in the current study was improvements (baseline to 3 months) in KOOS knee pain. The secondary outcomes were the improvements in the other four KOOS subscales, Activity-of-Daily-Living (ADL), Symptoms, Sports and Recreation (Sport/Rec), and Quality-of-Life (QoL). Data were treated as per the instructions provided by Roos and colleagues [
25], and all KOOS subscale scores were calculated from 0 (worst) to 100 (best).
2.5. Sample Size
The systematic review by Collins and colleagues [
26] indicated that the typical KOOS Pain score for elderly individuals with knee-OA is 50.7 (±17.9). We theorised that our intervention would lead to at least a moderate improvement in KOOS Pain, i.e., the intervention would have a moderate effect size of at least 0.6 [
27]. To have a moderate effect size of 0.6, the typical KOOS Pain score of 50.7 [
26] would need to increase by around 10 points (i.e., to 60.7).
The (estimated) within-group standard deviation is used in sample size estimations (for a given alpha) in an unpaired test. If the proposed comparison is a paired test then, assuming there is some within-subject correlation, the power of the test will be higher. As the statistical analysis used in the current study accounted for the within-subject correlations, when undertaking our sample size estimation, we shrank the standard deviation that was reported in the review by Collins and colleagues (±17.9) to two-thirds of their value, i.e., to ±12. Hence, using the Pain score of 50.7 (with an SD of ±12) and assuming an alpha of
p = 0.05 and power of 0.80, we estimated [
27] that two groups of 23 participants would be needed to detect a 10-point difference. As a pilot study, we did not adjust alpha to account for multiple testing.
2.6. Randomisation, Intervention and Evaluation
All participants completed a KOOS at week one and week four following gaining of consent (baseline-1, baseline-2). Once participants had completed the KOOS at baseline-1, they were asked to make a second appointment to see Doctor Bhattacharjee four weeks later. They were asked to complete the baseline-2 KOOS questionnaire immediately prior to attending this second appointment. At this appointment, participants were randomly assigned (1:1 allocation), using the allocation concealment approach (“sealed envelope”), to either an intervention or control group. The sealed envelopes had been supplied to Doctor Bhattacharjee by the study’s statistician (author AJS). The envelopes were labelled from 1 to 55, and inside each one there was a single piece of paper with the word “Control” or “Intervention” written on it. The envelopes were chronologically assigned to patients in the order they were seen by Doctor Bhattacharjee (1 to 55). When each envelope was opened, the piece of paper inside it indicated which group the patient should be assigned to. Those assigned to the intervention group were given an instruction document (see New sleeping position instructions,
Online Resource S2) that provided details of the new sleeping position (including a picture of the feet and lower legs of someone lying in this position) and what was involved in adopting this new sleeping position (i.e., instructions on how to keep the feet warm, etc). Participants were encouraged to ask questions for clarification. The instruction document indicated that participants should adopt the new sleeping position from that day forwards and maintain it for 3 months, and to complete and return a KOOS questionnaire at the end of this period. Participants were told that, if for any reason they could not adopt the new position, or they were unable to maintain the new sleeping position for three months, they should indicate this in writing on the questionnaire they returned (at 3 months). Those assigned to the control group were told nothing about the intervention and were simply told the study was about understanding knee pain in those with knee-OA. They, too, were asked to complete and return a final KOOS questionnaire, three months after completing the one at baseline-2.
Participants were provided with stamped addressed envelopes to use to return each questionnaire to another member of the research team (author JGB). JGB transcribed the responses from each questionnaire to a scoring Excel-file (KOOS website ‘koos.nu’). He was blinded as to which group each participant was assigned to.
Only two participants indicated they found it difficult to use the requested position. These two participants did not complete a third/final questionnaire, which meant their data could not be included in our statistical analysis. Given that we received positive comments, appended to the completed questionnaires, from several participants about the new sleeping position as well as indication from three participants that they had adopted the position on a permanent basis, this would suggest that adherence to the new sleeping position was high.
2.7. Statistical Analyses
Once all questionnaire responses for each participant had been transcribed into the scoring Excel-file, the average subscale scores for each participant at each time point (baseline-1, baseline-2, 3 months) were saved into database format and sent to the study’s statistician (author AJS) for analysis. The data were analysed using random effects regression modelling (StataCorp, College Station, TX, USA). Random effects modelling can loosely be thought of as an extension of the repeated-measures ANOVA method (see Everitt 2003 Modern Medical Statistics: a Practical Guide). The essential feature of such a model is that it takes account of the fact that readings for a particular individual are likely to be correlated. Regression models do not require the data to be normally distributed. The key assumption is that the residuals are normally, and identically, distributed. The models used were inspected to ensure that these assumptions were reasonably met. A second consideration is that all participants had three repeated measures. The random-effects regression modelling approach was chosen because it takes account of within-subject correlations.
Data were analysed using the following factors:
Group: two levels (intervention, control)
Assessment time: three levels (baseline-1, baseline-2, 3 months)
The only random factor used was participant: coded from 1–55.
The focus of the statistical modelling was to determine the difference between the intervention group compared to the control group, in improvements in KOOS outcomes at 3 months compared to baseline-1. Our analyses also evaluated if there were any group differences in KOOS scores at baseline-1 and if there were any differences in KOOS scores between groups at baseline-2. Finally, as this was a small-scale (preliminary) trial, improvements in KOOS outcomes at 3 months compared to baseline-1 were also evaluated separately for each group. Level of significance was accepted at p < 0.05
3. Results
There were no significant differences between the groups in any of the KOOS subscales at initial baseline (baseline-1), and there were no significant changes in these scores at baseline-2 (
p > 0.2). The five KOOS subscale outcomes for the intervention and control groups at both baseline time points and at 3 months following baseline are presented in
Figure 3. The output from our statistical analyses is shown in
Table 2. Note, rather than absolute values, the data in
Table 2 show the improvements at 3 months in KOOS scores from baseline-1 in the intervention group compared to the control group (i.e., the group-by-time interactions).
Figure 3 highlights that there was little change in KOOS outcomes across the three time points in the control group but there were noticeable improvements in KOOS outcomes in the intervention group at 3 months (i.e., post intervention) compared to baseline (1 and 2). The group-by-time interactions, however, were only statistically significant for KOOS subscale ADL (by 12.2 [5.3],
p = 0.02), although the one for QoL had an effect of moderate, but not statistically significant, magnitude (
p = 0.08,
Table 2).
When evaluating KOOS outcomes separately for each group, we found no differences in any of the KOOS subscales at 3 months compared to baseline in the control group. In contrast, KOOS sub-scale outcomes (except Sport/Rec) improved at 3 months in the intervention group compared to baseline-1 (group mean improvements: Pain from 50 to 62,
p = 0.002; Symptoms from 52 to 62
p = 0.023; ADL from 54 to 66,
p = 0.021; QoL from 31 to 44,
p = 0.047).
Figure 3.
Group mean (+SD) KOOS subscales at baseline-1 (start of study), baseline-2 (1 month following baseline-1) and at 3 months (either 3 months after changing sleeping position, intervention group; or 3 months after baseline-2, control group). * indicates significantly different to baseline-1 when analysing data for each group separately; ^ indicates significant group-by-time interaction: p < 0.05.
Figure 3.
Group mean (+SD) KOOS subscales at baseline-1 (start of study), baseline-2 (1 month following baseline-1) and at 3 months (either 3 months after changing sleeping position, intervention group; or 3 months after baseline-2, control group). * indicates significantly different to baseline-1 when analysing data for each group separately; ^ indicates significant group-by-time interaction: p < 0.05.
Table 2.
Differences in improvements from baseline-1 in the intervention group compared to control group, in KOOS scores (±SE) with 95% confidence intervals (CI) and the associated p-values.
Table 2.
Differences in improvements from baseline-1 in the intervention group compared to control group, in KOOS scores (±SE) with 95% confidence intervals (CI) and the associated p-values.
| | Mean Improvement in KOOS Sub-Scale | 95% CI | p-Value |
|---|
| Pain | 6.8 (5.7) | −4.5–18.0 | 0.24 |
| Symptom | 4.3 (5.3) | −6.0–14.7 | 0.41 |
| ADL | 12.2 (5.3) | 1.9–22.5 | 0.02 |
| Sport/Rec | 10.7 (6.9) | −2.9–24.3 | 0.12 |
| QoL | 10.0 (5.8) | −1.4–21.4 | 0.08 |
4. Discussion
We conducted a pilot trial to evaluate, in patients with knee-OA who routinely sleep supine, whether the intervention of changing sleeping position so the feet hang over the end of the mattress would ameliorate the knee pain and the associated physical problems they suffer. Results indicate that the intervention of sleeping with the feet hanging over the end of the mattress led to an improvement in KOOS Knee Pain (primary outcome, which improved by 6.8 score points) and to three of the other four KOOS subscales (including Activities-of-Daily-Living, which improved by 12.2 score points). However, when evaluating improvements in the intervention group in comparison to the control group (i.e., the group-by-time interactions), improvements in the primary outcome Knee Pain did not reach statistical significance; though improvements in the secondary outcome Activities-of-Daily-Living did, and those for Quality-of-Life showed a moderate (non-significant) effect (
Table 2). Overall, these favourable findings suggest that, for those who routinely sleep supine who suffer from knee-OA, this simple intervention could help ameliorate some of the associated physical problems they suffer.
Work from our lab has shown that when lying supine on a solid support, an external knee extension torque is induced that pushes the knee in full extension, but the induced knee torque is reduced to zero if a person changes their lying position so that the feet hang over the end of the support ([
19], also see
Figure 1). This would suggest that the favourable outcomes of the present study, were due to the new sleeping position (sleeping with feet over the end of the mattress) reducing the amount of time participants’ knees were ‘pushed’ into/held in full extension. This highlights that future work should determine the deleterious effects on knee structures caused by the knee being held in full extension for prolonged periods. Previous research has shown that normative spinal loading through the course of a day causes a decrease in the size (thickness) of the intervertebral discs [
28,
29], as fluid is “squeezed” from the disc [
30,
31]. Lying down to sleep unloads the spine and allows the intervertebral discs to rehydrate, and as a consequence, they regain volume (and thickness) [
28,
29]. This is why stature can be 1–2 cm greater at the beginning compared to end of the day [
32]. One might assume that lying down to sleep should also unload knee structures. It may be that having the knee fully unloaded allows an optimum supply of fluid (blood and nutrients) into knee cartilage and this “optimum” nutrient supply may be important in preventing chondrocyte death and/or matrix loss in knee cartilage or may aid the repair of such death/loss if it occurs? As highlighted above, chondrocyte death and matrix loss in knee cartilage have been shown to be a consequence of prolonged static knee loading in animal models [
7,
8] and both have been shown to be factors in the onset and progression of knee osteoarthritis [
9,
10]. It is possible that having the knee held in full extension when lying supine partially loads knee structures: though future research is required to confirm this. If future research does show that knee structures are partially loaded when the knee is held in full extension, then this would suggest that individuals who routinely sleep supine and regularly overload their knees, might be considered as high risk of experiencing chronic knee pain and ultimately developing knee-OA, e.g., sports men/women [
33], those involved in professions involving prolonged standing [
11,
12], or obese individuals [
34,
35]. Based on the findings of the current study, such individuals should be encouraged to adopt the sleeping position of lying with feet over the end of the mattress, in order to prevent the knee experiencing prolonged periods of it being ‘pushed’ into/held in full extension. The intervention could also be considered for those individuals who routinely sleep on their backs who are awaiting knee-replacement surgery, as it could make their wait for surgery more bearable.
Previous research has shown that the typical KOOS Pain score for elderly individuals with knee-OA is 50.7 (±17.9) [
26]. The participants in the present study had KOOS Pain scores at baseline of 49.4 (±16.2) and 50.0 (±18.2) for the intervention and control group, respectively. Participant scores for the four other KOOS subscales are also similar to typical scores [
26]. This indicates that the current study’s participants were typical of the wider knee-OA population, and thus, this suggests that this simple intervention should have similar effects in the wider knee-OA population for those who routinely sleep supine.
It is worth highlighting the practical aspects of the intervention (of lying supine with the feet hanging over the end of the mattress). Because the intervention requires a person to move further down the mattress so the feet can hang over the end of it, they will be unable to share a double-quilt/bed covers if they are sharing their bed with a spouse/partner. In addition, they will also have to use an extra blanket(s) to cover their feet to keep them warm. Furthermore, the intervention cannot be adopted by anyone who has a “kickboard” (or similar obstacle) at the end of their bed because such would prevent the individual from sleeping with their feet over the end of the mattress. However, based on the favourable outcomes found in the present study, mattress manufacturers should be encouraged to produce a mattress that has a “step-down” or downward slope at the foot end in which the feet could be placed when lying supine, which would avoid the above-highlighted problems. Use of such a mattress may also improve compliance to the intervention and thus its use may increase the efficacy of the intervention.
Another aspect of the intervention that should be highlighted is the seemingly high loss to follow-up (~17%, i.e., 9 out of 51). However, three of these losses (~6%) were due to the participants undergoing knee surgery, and a further two were due to participants (unintendingly) returning corrupted KOOS questionnaires. This highlights that only four out of the 51 (~8%) participants were lost/left the study without good cause. This would suggest that the intervention generally has good feasibility/acceptability.
Limitations
The limitations of this study include the following:
There was a slight improvement in Knee Pain in controls at 3 months that may have been due to a placebo effect, whereby participants had an altered perception of their pain simply because of being part of a research study. This would explain why the improvements in Knee Pain in the intervention group did not reach statistical significance in comparison to the control group even though there was a 12 score-point improvement at 3 months in this outcome measure in the intervention group.
We were unable to determine the radiological stage of OA (Kellgren-Lawrence staging) of each of the study’s participants. Ideally, both the intervention and control groups should have similar distribution of OA radiological grade. However, the study was undertaken in a General Practice, and thus recruiting patients with the same radiological criteria of osteoarthritis in each arm would have been difficult to achieve within a workable timeframe. An “allocation concealment” approach was used to randomly recruit patients into either the intervention or control group. Such random allocation, which was blinded to the clinician overseeing participant recruitment, would have minimised the likelihood of the two groups having significantly different distribution of OA radiological grade. The participant characteristics (
Table 1) indicate that the two groups were well matched in terms of mean OA severity (determined from the X-rays), which suggests that the two groups had a similar distribution of OA radiological grades.
Participants in the intervention group were obviously, not “blinded” to the intervention. However, it is widely recognised that blinding is not possible in trials that assess things like physiotherapy or behavioural interventions, because patients know what treatment they are receiving [
36], and this lack of blinding is not unusual for non-pharmacological intervention trials carried out in OA patients [
37].
We did not objectively determine if the new sleeping position was adopted, how long/often it was maintained during a night, or whether it was routinely continued for 3 months. However, all but two participants indicated they were able to adopt the new sleeping position. Nevertheless, we recognise that participants would not have spent the whole night in the new position and may have only maintained it for intermittent periods throughout the night. Thus, another limitation is that we are unable to say how long or how often during a night the new sleeping position should be maintained in order to have a positive effect.
A final limitation is the relatively small sample size. The limitations of having a small sample size are well known (e.g., see [
38]), and are related to the potential of data from one or two participants having a disproportional effect on group trends, and the increased risk of Type II error (accepting a null hypothesis that is false). However, the present study identified evidence of a beneficial effect of the intervention and, given the rigorous statistical analyses used, this effect was not due to the influence of one or two outliers. Nevertheless, given the small sample size, the findings should be considered as preliminary.
The favourable findings of the study suggest that future work is needed to determine the specific effects on knee structures caused by the knee being held in full extension. Finally, finding a significant effect for the secondary outcome, but not for the primary outcome (even though the primary outcome improved), indicates that a larger study is warranted to fully assess the efficacy of this simple intervention.