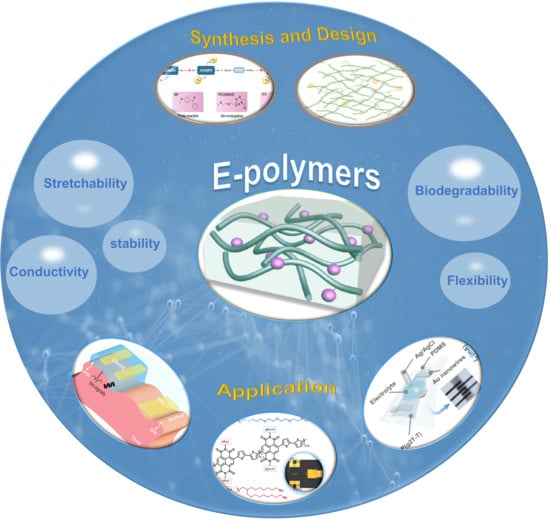
E-Polymers: Applications in Biological Interfaces and Organisms
Abstract
:1. Introduction
2. Synthesis and Design of E-Polymers

3. Properties of E-Polymers
4. Engineering Material Structure
4.1. 3D Structure
4.2. Serpentine Structure
4.3. Microstructure
4.4. Mesh and Fiber Structure
5. E-Polymers for Bio-Integrated Applications
5.1. Pressure Sensor
5.2. Biosensor
5.3. Energy Harvester
5.4. Strategies for Self-Powered Intelligent Sensing Systems
6. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Solomon, D.H.; Rudin, R.S. Digital health technologies: Opportunities and challenges in rheumatology. Nat. Rev. Rheumatol. 2020, 16, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Celik, A.; Romdhane, I.; Kaddoum, G.; Eltawil, A.M. A Top-Down Survey on Optical Wireless Communications for the Internet of Things. IEEE Commun. Surv. Tutor. 2023, 25, 1–45. [Google Scholar] [CrossRef]
- Filieri, R.; Acikgoz, F.; Du, H. Electronic word-of-mouth from video bloggers: The role of content quality and source homophily across hedonic and utilitarian products. J. Bus. Res. 2023, 160, 113774. [Google Scholar] [CrossRef]
- Leng, Z.; Zhu, P.; Wang, X.; Wang, Y.; Li, P.; Huang, W.; Li, B.; Jin, R.; Han, N.; Wu, J.; et al. Sebum-Membrane-Inspired Protein-Based Bioprotonic Hydrogel for Artificial Skin and Human-Machine Merging Interface. Adv. Funct. Mater. 2023, 33, 2211056. [Google Scholar] [CrossRef]
- Cao, W.; Mao, H.; McCallum, N.C.; Zhou, X.; Sun, H.; Sharpe, C.; Korpanty, J.; Hu, Z.; Ni, Q.Z.; Burkart, M.D.; et al. Biomimetic pheomelanin to unravel the electronic, molecular and supramolecular structure of the natural product. Chem. Sci. 2023, 14, 4183–4192. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Oh, J.Y.; Xu, J.; Tran, H.; Bao, Z. Skin-Inspired Electronics: An Emerging Paradigm. Acc. Chem. Res. 2018, 51, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Dai, Y.; Zhao, Z.; Xia, F.; Li, Y.; Liu, Y.; Cheng, P.; Strzalka, J.; Li, S.; Li, N.; et al. Intrinsically stretchable neuromorphic devices for on-body processing of health data with artificial intelligence. Matter 2022, 5, 3375–3390. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Lu, H.; Li, S.; Zhang, Y. Electronic fibers and textiles: Recent progress and perspective. iScience 2021, 24, 102716. [Google Scholar] [CrossRef]
- McCulloch, I.; Chabinyc, M.; Brabec, C.; Nielsen, C.B.; Watkins, S.E. Sustainability considerations for organic electronic products. Nat. Mater. 2023, 22, 1304–1310. [Google Scholar] [CrossRef]
- Balakrishnan, G.; Song, J.; Mou, C.; Bettinger, C.J. Recent Progress in Materials Chemistry to Advance Flexible Bioelectronics in Medicine. Adv. Mater. 2022, 34, 2106787. [Google Scholar] [CrossRef]
- Ishijima, Y.; Imai, H.; Oaki, Y. Tunable Mechano-responsive Color-Change Properties of Organic Layered Material by Intercalation. Chem 2017, 3, 509–521. [Google Scholar] [CrossRef]
- Yoon, J.; Hou, Y.; Knoepfel, A.M.; Yang, D.; Ye, T.; Zheng, L.; Yennawar, N.; Sanghadasa, M.; Priya, S.; Wang, K. Bio-inspired strategies for next-generation perovskite solar mobile power sources. Chem. Soc. Rev. 2021, 50, 12915–12984. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Jiang, Y.; Bao, Z.; Yan, X. Skin-Inspired Electronics Enabled by Supramolecular Polymeric Materials. CCS Chem. 2019, 1, 431–447. [Google Scholar] [CrossRef]
- Glaudell, A.M.; Cochran, J.E.; Patel, S.N.; Chabinyc, M.L. Impact of the Doping Method on Conductivity and Thermopower in Semiconducting Polythiophenes. Adv. Energy Mater. 2015, 5, 1401072. [Google Scholar] [CrossRef]
- Electronic Materials Editorial Office. Acknowledgment to Reviewers of Electronic Materials in 2021. Electron. Mater. 2022, 3, 63–64. [Google Scholar] [CrossRef]
- Chortos, A.; Liu, J.; Bao, Z. Pursuing prosthetic electronic skin. Nat. Mater. 2016, 15, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, X.; Sarkar, B.; Gagnon-Lafrenais, N.; Cicoira, F. Recent Progress on Self-Healable Conducting Polymers. Adv. Mater. 2022, 34, 2108932. [Google Scholar] [CrossRef] [PubMed]
- Won, D.; Bang, J.; Choi, S.H.; Pyun, K.R.; Jeong, S.; Lee, Y.; Ko, S.H. Transparent Electronics for Wearable Electronics Application. Chem. Rev. 2023, 123, 9982–10078. [Google Scholar] [CrossRef]
- Someya, T.; Amagai, M. Toward a new generation of smart skins. Nat. Biotechnol. 2019, 37, 382–388. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Z.; Wang, H.; Jin, B.; Meng, C.; Chen, X.; Li, R.; Wang, H.; Xin, M.; Zhao, Z.; et al. Self-Healing, Reconfigurable, Thermal-Switching, Transformative Electronics for Health Monitoring. Adv. Mater. 2023, 35, 2207742. [Google Scholar] [CrossRef]
- Chang, B.; Ai, Z.; Shi, D.; Zhong, Y.; Zhang, K.; Shao, Y.; Zhang, L.; Shen, J.; Wu, Y.; Hao, X. p–n tungsten oxide homojunctions for Vis-NIR light-enhanced electrocatalytic hydrogen evolution. J. Mater. Chem. A 2019, 7, 19573–19580. [Google Scholar] [CrossRef]
- Sun, L.; Cheng, C.; Wang, S.; Tang, J.; Xie, R.; Wang, D. Bioinspired, Nanostructure-Amplified, Subcutaneous Light Harvesting to Power Implantable Biomedical Electronics. ACS Nano 2021, 15, 12475–12482. [Google Scholar] [CrossRef] [PubMed]
- Janata, J.; Josowicz, M. Conducting polymers in electronic chemical sensors. Nat. Mater. 2003, 2, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Abdelsamie, M.A.; Chaney, T.P.; Yan, H.; Schneider, S.A.; Ayhan, I.A.; Gomez, E.D.; Reynolds, J.R.; Toney, M.F. Revealing temperature-dependent polymer aggregation in solution with small-angle X-ray scattering. J. Mater. Chem. A 2022, 10, 2096–2104. [Google Scholar] [CrossRef]
- Li, W.; Liu, Q.; Zhang, Y.; Li, C.; He, Z.; Choy, W.C.H.; Low, P.J.; Sonar, P.; Kyaw, A.K.K. Biodegradable Materials and Green Processing for Green Electronics. Adv. Mater. 2020, 32, 2001591. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Kuang, X.; Deng, J.; Wang, Y.; Wang, A.C.; Ding, W.; Lai, Y.; Chen, J.; Wang, P.; Lin, Z.; et al. Shape Memory Polymers for Body Motion Energy Harvesting and Self-Powered Mechanosensing. Adv. Mater. 2018, 30, 1705195. [Google Scholar] [CrossRef] [PubMed]
- George, P.M.; LaVan, D.A.; Burdick, J.A.; Chen, C.-Y.; Liang, E.; Langer, R. Electrically Controlled Drug Delivery from Biotin-Doped Conductive Polypyrrole. Adv. Mater. 2006, 18, 577–581. [Google Scholar] [CrossRef]
- Jung, M.R.; Horgen, F.D.; Orski, S.V.; Rodriguez, C.V.; Beers, K.L.; Balazs, G.H.; Jones, T.T.; Work, T.M.; Brignac, K.C.; Royer, S.-J.; et al. Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms. Mar. Pollut. Bull. 2018, 127, 704–716. [Google Scholar] [CrossRef]
- Kobayashi, M. Adhesive Polymers Inspired by Marine Sessile Organisms. Sen’i Gakkaishi 2017, 73, P505–P508. [Google Scholar] [CrossRef]
- Loher, S.; Schneider, O.D.; Maienfisch, T.; Bokorny, S.; Stark, W.J. Micro-Organism-Triggered Release of Silver Nanoparticles from Biodegradable Oxide Carriers Allows Preparation of Self-Sterilizing Polymer Surfaces. Small 2008, 4, 824–832. [Google Scholar] [CrossRef]
- Sun, H.; Guo, X.; Facchetti, A. High-Performance n-Type Polymer Semiconductors: Applications, Recent Development, and Challenges. Chem 2020, 6, 1310–1326. [Google Scholar] [CrossRef]
- Wu, H.-C.; Hung, C.-C.; Hong, C.-W.; Sun, H.-S.; Wang, J.-T.; Yamashita, G.; Higashihara, T.; Chen, W.-C. Isoindigo-Based Semiconducting Polymers Using Carbosilane Side Chains for High Performance Stretchable Field-Effect Transistors. Macromolecules 2016, 49, 8540–8548. [Google Scholar] [CrossRef]
- Mooney, M.; Nyayachavadi, A.; Rondeau-Gagné, S. Eco-friendly semiconducting polymers: From greener synthesis to greener processability. J. Mater. Chem. C 2020, 8, 14645–14664. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, Z.; Wang, Y.; Meng, Z.; Zhao, Z. A self-healing carboxymethyl chitosan/oxidized carboxymethyl cellulose hydrogel with fluorescent bioprobes for glucose detection. Carbohydr. Polym. 2021, 274, 118642. [Google Scholar] [CrossRef]
- Zarei, M.; Lee, G.; Lee, S.G.; Cho, K. Advances in Biodegradable Electronic Skin: Material Progress and Recent Applications in Sensing, Robotics, and Human–Machine Interfaces. Adv. Mater. 2023, 35, 2203193. [Google Scholar] [CrossRef]
- Ates, H.C.; Nguyen, P.Q.; Gonzalez-Macia, L.; Morales-Narváez, E.; Güder, F.; Collins, J.J.; Dincer, C. End-to-end design of wearable sensors. Nat. Rev. Mater. 2022, 7, 887–907. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Lin, M.; Yin, L.; De la Paz, E.; Pei, K.; Sonsa-Ard, T.; de Loyola Silva, A.N.; Khorshed, A.A.; Zhang, F.; Tostado, N.; et al. An epidermal patch for the simultaneous monitoring of haemodynamic and metabolic biomarkers. Nat. Biomed. Eng. 2021, 5, 737–748. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, Z.; Li, W.; Zhang, H.; Dai, G.; Zhou, X. Research progress of self-healing polymer materials for flexible electronic devices. J. Polym. Sci. 2023, 61, 1554–1571. [Google Scholar] [CrossRef]
- Hao, T.; Wang, S.; Xu, H.; Zhang, X.; Xue, J.; Liu, S.; Song, Y.; Li, Y.; Zhao, J. Stretchable electrochromic devices based on embedded WO3@AgNW Core-Shell nanowire elastic conductors. Chem. Eng. J. 2021, 426, 130840. [Google Scholar] [CrossRef]
- Rao, Z.; Ershad, F.; Almasri, A.; Gonzalez, L.; Wu, X.; Yu, C. Soft Electronics for the Skin: From Health Monitors to Human–Machine Interfaces. Adv. Mater. Technol. 2020, 5, 2000233. [Google Scholar] [CrossRef]
- Hanif, A.; Ghosh, G.; Meeseepong, M.; Chouhdry, H.H.; Bag, A.; Chinnamani, M.V.; Kumar, S.; Sultan, M.J.; Yadav, A.; Lee, N.-E. A Composite Microfiber for Biodegradable Stretchable Electronics. Micromachines 2021, 12, 1036. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, K.; Zhang, Q.; Yu, S.S.; Han, B.S.; Wang, J.; Zhao, M.; Meng, X.; Chen, S.; Zheng, Y. Flexible integrated sensor with asymmetric structure for simultaneously 3D tactile and thermal sensing. Biosens. Bioelectron. 2023, 224, 115054. [Google Scholar] [CrossRef]
- He, F.; You, X.; Wang, W.; Bai, T.; Xue, G.; Ye, M. Recent Progress in Flexible Microstructural Pressure Sensors toward Human–Machine Interaction and Healthcare Applications. Small Methods 2021, 5, 2001041. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, S.; Cai, R.; Tan, W. Rapid water-responsive shape memory films for smart resistive bending sensors. Nano Today 2021, 38, 101202. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Han, Z.; Wang, J.; Zou, X. A system for measuring borehole diametric deformation based on mechanical contact and micro-optical imaging. Measurement 2018, 130, 191–197. [Google Scholar] [CrossRef]
- Luan, H.; Zhang, Q.; Liu, T.-L.; Wang, X.; Zhao, S.; Wang, H.; Yao, S.; Xue, Y.; Kwak, J.W.; Bai, W.; et al. Complex 3D microfluidic architectures formed by mechanically guided compressive buckling. Sci. Adv. 2021, 7, eabj3686. [Google Scholar] [CrossRef]
- Miao, J.; Wang, Y.; Liu, J.; Wang, L. Organoboron molecules and polymers for organic solar cell applications. Chem. Soc. Rev. 2022, 51, 153–187. [Google Scholar] [CrossRef]
- Osaka, I.; Takimiya, K. Naphthobischalcogenadiazole Conjugated Polymers: Emerging Materials for Organic Electronics. Adv. Mater. 2017, 29, 1605218. [Google Scholar] [CrossRef]
- Sailor, M.J.; Klavetter, F.L.; Grubbs, R.H.; Lewis, N.S. Electronic properties of junctions between silicon and organic conducting polymers. Nature 1990, 346, 155–157. [Google Scholar] [CrossRef]
- Qiao, Z.; Shen, M.; Xiao, Y.; Zhu, M.; Mignani, S.; Majoral, J.-P.; Shi, X. Organic/inorganic nanohybrids formed using electrospun polymer nanofibers as nanoreactors. Coord. Chem. Rev. 2018, 372, 31–51. [Google Scholar] [CrossRef]
- Clothier, G.K.K.; Guimarães, T.R.; Thompson, S.W.; Rho, J.Y.; Perrier, S.; Moad, G.; Zetterlund, P.B. Multiblock copolymer synthesis via RAFT emulsion polymerization. Chem. Soc. Rev. 2023, 52, 3438–3469. [Google Scholar] [CrossRef]
- Kim, J.; Lee, K.H.; Lee, J.Y. Extracting Polaron Recombination from Electroluminescence in Organic Light-Emitting Diodes by Artificial Intelligence. Adv. Mater. 2023, 35, 2209953. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y. Insight into conjugated polymers for organic electrochemical transistors. Trends Chem. 2023, 5, 279–294. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Wang, T.; Hao, L.; Lin, E.; Chen, Y.; Cheng, P.; Zhang, Z. Organic flux synthesis of covalent organic frameworks. Chem 2023, 9, 2178–2193. [Google Scholar] [CrossRef]
- Liang, S.; Xiao, C.; Xie, C.; Liu, B.; Fang, H.; Li, W. 13% Single-Component Organic Solar Cells based on Double-Cable Conjugated Polymers with Pendent Y-Series Acceptors. Adv. Mater. 2023, 35, 2300629. [Google Scholar] [CrossRef]
- Wang, Y.; He, Q.; Wang, Z.; Zhang, S.; Li, C.; Wang, Z.; Park, Y.-L.; Cai, S. Liquid Crystal Elastomer Based Dexterous Artificial Motor Unit. Adv. Mater. 2023, 35, 2211283. [Google Scholar] [CrossRef]
- Li, J.; Qian, Y.; Li, W.; Yu, S.; Ke, Y.; Qian, H.; Lin, Y.; Hou, C.; Shyue, J.; Zhou, J.; et al. Polymeric Memristor Based Artificial Synapses with Ultra-Wide Operating Temperature. Adv. Mater. 2023, 35, 2209728. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, Q.; Shen, J.; Li, H.; Zhang, Y.; Chen, W.; Yu, S.; Li, X.; Chen, Y. Emerging tetrapyrrole porous organic polymers for chemosensing applications. Coord. Chem. Rev. 2023, 482, 215078. [Google Scholar] [CrossRef]
- Li, N.; Dai, Y.; Li, Y.; Dai, S.; Strzalka, J.; Su, Q.; De Oliveira, N.; Zhang, Q.; St. Onge, P.B.J.; Rondeau-Gagné, S.; et al. A universal and facile approach for building multifunctional conjugated polymers for human-integrated electronics. Matter 2021, 4, 3015–3029. [Google Scholar] [CrossRef]
- Wu, J.; Wang, N.; Xie, Y.-R.; Liu, H.; Huang, X.; Cong, X.; Chen, H.-Y.; Ma, J.; Liu, F.; Zhao, H.; et al. Polymer-like Inorganic Double Helical van der Waals Semiconductor. Nano Lett. 2022, 22, 9054–9061. [Google Scholar] [CrossRef]
- Mahenderkar, N.K.; Chen, Q.; Liu, Y.-C.; Duchild, A.R.; Hofheins, S.; Chason, E.; Switzer, J.A. Epitaxial lift-off of electrodeposited single-crystal gold foils for flexible electronics. Science 2017, 355, 1203–1206. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Z.; Wang, Y.-X.; Li, D.; Coen, C.-T.; Hwaun, E.; Chen, G.; Wu, H.-C.; Zhong, D.; Niu, S.; et al. Topological supramolecular network enabled high-conductivity, stretchable organic bioelectronics. Science 2022, 375, 1411–1417. [Google Scholar] [CrossRef]
- Feng, K.; Shan, W.; Wang, J.; Lee, J.; Yang, W.; Wu, W.; Wang, Y.; Kim, B.J.; Guo, X.; Guo, H. Cyano-Functionalized n-Type Polymer with High Electron Mobility for High-Performance Organic Electrochemical Transistors. Adv. Mater. 2022, 34, 2201340. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Gao, C.; Ni, Z.; Zhang, X.; Hu, W.; Dong, H. Recent advances in n-type and ambipolar organic semiconductors and their multi-functional applications. Chem. Soc. Rev. 2023, 52, 1331–1381. [Google Scholar] [CrossRef]
- Shen, T.; Li, W.; Zhao, Y.; Wang, Y.; Liu, Y. A Hybrid Acceptor-Modulation Strategy: Fluorinated Triple-Acceptor Architecture for Significant Enhancement of Electron Transport in High-Performance Unipolar n-Type Organic Transistors. Adv. Mater. 2023, 35, 2210093. [Google Scholar] [CrossRef]
- Li, Y.; Huang, E.; Guo, X.; Feng, K. Cyano-functionalized organic and polymeric semiconductors for high-performance n-type organic electronic devices. Mater. Chem. Front. 2023, 7, 3803–3819. [Google Scholar] [CrossRef]
- Wang, S.; Xu, J.; Wang, W.; Wang, G.-J.N.; Rastak, R.; Molina-Lopez, F.; Chung, J.W.; Niu, S.; Feig, V.R.; Lopez, J.; et al. Skin electronics from scalable fabrication of an intrinsically stretchable transistor array. Nature 2018, 555, 83–88. [Google Scholar] [CrossRef]
- Ochs, J.; Pagnacco, C.A.; Barroso-Bujans, F. Macrocyclic polymers: Synthesis, purification, properties and applications. Prog. Polym. Sci. 2022, 134, 101606. [Google Scholar] [CrossRef]
- Kim, J.H.; Kang, D.W.; Yun, H.; Kang, M.; Singh, N.; Kim, J.S.; Hong, C.S. Post-synthetic modifications in porous organic polymers for biomedical and related applications. Chem. Soc. Rev. 2022, 51, 43–56. [Google Scholar] [CrossRef]
- Korshak, Y.V.; Medvedeva, T.V.; Ovchinnikov, A.A.; Spector, V.N. Organic polymer ferromagnet. Nature 1987, 326, 370–372. [Google Scholar] [CrossRef]
- Lin, Z.; Kabe, R.; Nishimura, N.; Jinnai, K.; Adachi, C. Organic Long-Persistent Luminescence from a Flexible and Transparent Doped Polymer. Adv. Mater. 2018, 30, 1803713. [Google Scholar] [CrossRef]
- Luo, D.; Li, M.; Ma, Q.; Wen, G.; Dou, H.; Ren, B.; Liu, Y.; Wang, X.; Shui, L.; Chen, Z. Porous organic polymers for Li-chemistry-based batteries: Functionalities and characterization studies. Chem. Soc. Rev. 2022, 51, 2917–2938. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, C.; Alessandri, R.; Diroll, B.T.; Li, Y.; Liang, H.; Fan, X.; Wang, K.; Cho, H.; Liu, Y.; et al. High-efficiency stretchable light-emitting polymers from thermally activated delayed fluorescence. Nat. Mater. 2023, 22, 737–745. [Google Scholar] [CrossRef]
- Kohlman, R.S.; Joo, J.; Min, Y.G.; MacDiarmid, A.G.; Epstein, A.J. Crossover in Electrical Frequency Response through an Insulator-Metal Transition. Phys. Rev. Lett. 1996, 77, 2766–2769. [Google Scholar] [CrossRef]
- Ning, H.; Jiang, Q.; Han, P.; Lin, M.; Zhang, G.; Chen, J.; Chen, H.; Zeng, S.; Gao, J.; Liu, J.; et al. Manipulating the solubility properties of polymer donors for high-performance layer-by-layer processed organic solar cells. Energy Environ. Sci. 2021, 14, 5919–5928. [Google Scholar] [CrossRef]
- Noro, S.-I.; Kitagawa, S.; Akutagawa, T.; Nakamura, T. Coordination polymers constructed from transition metal ions and organic N-containing heterocyclic ligands: Crystal structures and microporous properties. Prog. Polym. Sci. 2009, 34, 240–279. [Google Scholar] [CrossRef]
- Yao, N.; Wang, J.; Chen, Z.; Bian, Q.; Xia, Y.; Zhang, R.; Zhang, J.; Qin, L.; Zhu, H.; Zhang, Y.; et al. Efficient Charge Transport Enables High Efficiency in Dilute Donor Organic Solar Cells. J. Phys. Chem. Lett. 2021, 12, 5039–5044. [Google Scholar] [CrossRef]
- Gao, W.; Yu, C. Wearable and Implantable Devices for Healthcare. Adv. Healthc. Mater. 2021, 10, 2101548. [Google Scholar] [CrossRef]
- Tao, K.; Makam, P.; Aizen, R.; Gazit, E. Self-assembling peptide semiconductors. Science 2017, 358, aam9756. [Google Scholar] [CrossRef]
- Ratcliff, E.L.; Shallcross, R.C.; Armstrong, N.R. Introduction: Electronic Materials. Chem. Rev. 2016, 116, 12821–12822. [Google Scholar] [CrossRef]
- Gumyusenge, A. Organic Iono-Electronics, a New Front for Semiconducting Polymers to Shine. Acc. Mater. Res. 2022, 3, 669–671. [Google Scholar] [CrossRef]
- Zhao, F.; Shi, Y.; Pan, L.; Yu, G. Multifunctional Nanostructured Conductive Polymer Gels: Synthesis, Properties, and Applications. Acc. Chem. Res. 2017, 50, 1734–1743. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Yan, L.; Zhao, X.; Chen, X.; Li, A.; Zheng, D.; Zhou, X.; Dai, X.; Xu, F.-J. Versatile Types of Organic/Inorganic Nanohybrids: From Strategic Design to Biomedical Applications. Chem. Rev. 2019, 119, 1666–1762. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Son, D.; Wang, G.-J.N.; Liu, Y.; Lopez, J.; Kim, Y.; Oh, J.Y.; Katsumata, T.; Mun, J.; Lee, Y.; et al. Tough and Water-Insensitive Self-Healing Elastomer for Robust Electronic Skin. Adv. Mater. 2018, 30, 1706846. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, N.; Liu, W.; Prominski, A.; Kang, S.; Dai, Y.; Liu, Y.; Hu, H.; Wai, S.; Dai, S.; et al. Achieving tissue-level softness on stretchable electronics through a generalizable soft interlayer design. Nat. Commun. 2023, 14, 4488. [Google Scholar] [CrossRef]
- Grądzka, E.; Wysocka-Żołopa, M.; Winkler, K. Fullerene-Based Conducting Polymers: n-Dopable Materials for Charge Storage Application. Adv. Energy Mater. 2020, 10, 2001443. [Google Scholar] [CrossRef]
- Li, J.; Qiao, J.; Lian, K. Hydroxide ion conducting polymer electrolytes and their applications in solid supercapacitors: A review. Energy Storage Mater. 2019, 24, 6–21. [Google Scholar] [CrossRef]
- Lutkenhaus, J. A radical advance for conducting polymers. Science 2018, 359, 1334–1335. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, W.; Kumar, R.; Kumar, M.; Zhang, J. Conducting polymer-based nanostructures for gas sensors. Coord. Chem. Rev. 2022, 462, 214517. [Google Scholar] [CrossRef]
- Le, T.-H.; Kim, Y.; Yoon, H. Electrical and Electrochemical Properties of Conducting Polymers. Polymers 2017, 9, 150. [Google Scholar] [CrossRef]
- Alessandri, R.; Sami, S.; Barnoud, J.; de Vries, A.H.; Marrink, S.J.; Havenith, R.W.A. Resolving Donor–Acceptor Interfaces and Charge Carrier Energy Levels of Organic Semiconductors with Polar Side Chains. Adv. Funct. Mater. 2020, 30, 2004799. [Google Scholar] [CrossRef]
- Shi, Y.; Pei, P.; Cheng, X.; Yan, Z.; Han, M.; Li, Z.; Gao, C.; Rogers, J.A.; Huang, Y.; Zhang, Y. An analytic model of two-level compressive buckling with applications in the assembly of free-standing 3D mesostructures. Soft Matter 2018, 14, 8828–8837. [Google Scholar] [CrossRef]
- Chang, J.-K.; Chang, H.-P.; Guo, Q.; Koo, J.; Wu, C.-I.; Rogers, J.A. Biodegradable Electronic Systems in 3D, Heterogeneously Integrated Formats. Adv. Mater. 2018, 30, 1704955. [Google Scholar] [CrossRef]
- Taylor, J.M.; Luan, H.; Lewis, J.A.; Rogers, J.A.; Nuzzo, R.G.; Braun, P.V. Biomimetic and Biologically Compliant Soft Architectures via 3D and 4D Assembly Methods: A Perspective. Adv. Mater. 2022, 34, 2108391. [Google Scholar] [CrossRef]
- Yan, Z.; Zhang, F.; Wang, J.; Liu, F.; Guo, X.; Nan, K.; Lin, Q.; Gao, M.; Xiao, D.; Shi, Y.; et al. 3D Assembly: Controlled Mechanical Buckling for Origami-Inspired Construction of 3D Microstructures in Advanced Materials. Adv. Funct. Mater. 2016, 26, 2586. [Google Scholar] [CrossRef]
- Han, M.; Guo, X.; Chen, X.; Liang, C.; Zhao, H.; Zhang, Q.; Bai, W.; Zhang, F.; Wei, H.; Wu, C.; et al. Submillimeter-scale multimaterial terrestrial robots. Sci. Robot. 2023, 7, eabn0602. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, H.; Han, W.; Lin, H.; Li, R.; Zhu, J.; Huang, W. 3D Printed Flexible Strain Sensors: From Printing to Devices and Signals. Adv. Mater. 2021, 33, 2004782. [Google Scholar] [CrossRef]
- Guo, X.; Wang, X.; Ou, D.; Ye, J.; Pang, W.; Huang, Y.; Rogers, J.A.; Zhang, Y. Controlled mechanical assembly of complex 3D mesostructures and strain sensors by tensile buckling. npj Flex. Electron. 2018, 2, 14. [Google Scholar] [CrossRef]
- Yan, Z.; Han, M.; Yang, Y.; Nan, K.; Luan, H.; Luo, Y.; Zhang, Y.; Huang, Y.; Rogers, J.A. Deterministic assembly of 3D mesostructures in advanced materials via compressive buckling: A short review of recent progress. Extrem. Mech. Lett. 2017, 11, 96–104. [Google Scholar] [CrossRef]
- Fan, Z.; Hwang, K.-C.; Rogers, J.A.; Huang, Y.; Zhang, Y. A double perturbation method of postbuckling analysis in 2D curved beams for assembly of 3D ribbon-shaped structures. J. Mech. Phys. Solids 2018, 111, 215–238. [Google Scholar] [CrossRef]
- Bashandeh, K.; Humood, M.; Lee, J.; Han, M.; Cui, Y.; Shi, Y.; Huang, Y.; Rogers, J.A.; Polycarpou, A.A. The effect of defects on the cyclic behavior of polymeric 3D kirigami structures. Extrem. Mech. Lett. 2020, 36, 100650. [Google Scholar] [CrossRef]
- Humood, M.; Shi, Y.; Han, M.; Lefebvre, J.; Yan, Z.; Pharr, M.; Zhang, Y.; Huang, Y.; Rogers, J.A.; Polycarpou, A.A. Fabrication and Deformation of 3D Multilayered Kirigami Microstructures. Small 2018, 14, 1703852. [Google Scholar] [CrossRef] [PubMed]
- Humood, M.; Lefebvre, J.; Shi, Y.; Han, M.; Fincher, C.D.; Pharr, M.; Rogers, J.A.; Polycarpou, A.A. Fabrication and Mechanical Cycling of Polymer Microscale Architectures for 3D MEMS Sensors. Adv. Eng. Mater. 2019, 21, 1801254. [Google Scholar] [CrossRef]
- Guo, X.; Xu, Z.; Zhang, F.; Wang, X.; Zi, Y.; Rogers, J.A.; Huang, Y.; Zhang, Y. Reprogrammable 3D Mesostructures through Compressive Buckling of Thin Films with Prestrained Shape Memory Polymer. Acta Mech. Solida Sin. 2018, 31, 589–598. [Google Scholar] [CrossRef]
- Park, Y.; Luan, H.; Kwon, K.; Zhao, S.; Franklin, D.; Wang, H.; Zhao, H.; Bai, W.; Kim, J.U.; Lu, W.; et al. Transformable, Freestanding 3D Mesostructures Based on Transient Materials and Mechanical Interlocking. Adv. Funct. Mater. 2019, 29, 1903181. [Google Scholar] [CrossRef]
- Rader, B.; Whaley, C.M.; Rogers, W.S.; Brownstein, J.S.; Cantor, J. Assessment of geographic access to monoclonal antibodies in the United States. J. Travel Med. 2022, 29, taac018. [Google Scholar] [CrossRef] [PubMed]
- Terrance, T.C.; Sugarwala, L.; McIntosh, S.; Bisbee-Burrows, M.; Castillejo, L.; Evans, A.; Reed, K.; Rogers, K.; Cullen, J.P. Structural racism in healthcare and research: A community-led model of curriculum development and implementation. J. Clin. Transl. Sci. 2023, 7, e18. [Google Scholar] [CrossRef]
- Fan, Z.; Yang, Y.; Zhang, F.; Xu, Z.; Zhao, H.; Wang, T.; Song, H.; Huang, Y.; Rogers, J.A.; Zhang, Y. Inverse Design Strategies for 3D Surfaces Formed by Mechanically Guided Assembly. Adv. Mater. 2020, 32, 1908424. [Google Scholar] [CrossRef]
- Abdullah, A.M.; Li, X.; Braun, P.V.; Rogers, J.A.; Hsia, K.J. Kirigami-Inspired Self-Assembly of 3D Structures. Adv. Funct. Mater. 2020, 30, 1909888. [Google Scholar] [CrossRef]
- Zhao, H.; Cheng, X.; Wu, C.; Liu, T.; Zhao, Q.; Li, S.; Ni, X.; Yao, S.; Han, M.; Huang, Y.; et al. Mechanically Guided Hierarchical Assembly of 3D Mesostructures. Adv. Mater. 2022, 34, 2109416. [Google Scholar] [CrossRef]
- Rogers, M.; Wattis, J.; Stephenson, J.; Khan, W.; Curran, S. A questionnaire-based study of attitudes to spirituality in mental health practitioners and the relevance of the concept of spiritually competent care. Int. J. Ment. Health Nurs. 2019, 28, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.; Park, Y.; Luan, H.W.; Dalgin, G.; Jeffris, K.; Yoon, H.J.; Chung, T.S.; Kim, J.U.; Kwak, S.S.; Lee, G.; et al. Transparent, Compliant 3D Mesostructures for Precise Evaluation of Mechanical Characteristics of Organoids. Adv. Mater. 2021, 33, 2100026. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, M.; Wegener, A.J.; Vázquez-Guardado, A.; Efimov, A.I.; Lie, F.; Wang, T.; Ma, Y.; Banks, A.; Li, Z.; et al. Preparation and use of wireless reprogrammable multilateral optogenetic devices for behavioral neuroscience. Nat. Protoc. 2022, 17, 1073–1096. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, Z.; Li, M.; Fang, L.; Chen, F.; Han, S.; Lan, L.; Chen, J.; Chen, Q.; Wang, H.; et al. High-Transconductance, Highly Elastic, Durable and Recyclable All-Polymer Electrochemical Transistors with 3D Micro-Engineered Interfaces. Nano-Micro Lett. 2022, 14, 184. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Wang, H.; Xue, Y.; Pan, Y.; Kim, J.-T.; Ni, X.; Liu, T.-L.; Yang, Y.; Han, M.; Huang, Y.; et al. A dynamically reprogrammable surface with self-evolving shape morphing. Nature 2022, 609, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Min, S.; Kim, D.H.; Joe, D.J.; Kim, B.W.; Jung, Y.H.; Lee, J.H.; Lee, B.; Doh, I.; An, J.; Youn, Y.; et al. Clinical Validation of a Wearable Piezoelectric Blood-Pressure Sensor for Continuous Health Monitoring. Adv. Mater. 2023, 35, 2301627. [Google Scholar] [CrossRef]
- Ok, J.; Park, S.; Jung, Y.H.; Kim, T. Wearable and Implantable Cortisol-Sensing Electronics for Stress Monitoring. Adv. Mater. 2023, e2211595. [Google Scholar] [CrossRef]
- Sun, F.; Jiang, H.; Wang, H.; Zhong, Y.; Xu, Y.; Xing, Y.; Yu, M.; Feng, L.-W.; Tang, Z.; Liu, J.; et al. Soft Fiber Electronics Based on Semiconducting Polymer. Chem. Rev. 2023, 123, 4693–4763. [Google Scholar] [CrossRef]
- Shi, L.; Li, Z.; Chen, M.; Zhu, T.; Wu, L. Ultrasensitive and Ultraprecise Pressure Sensors for Soft Systems. Adv. Mater. 2023, 35, 2210091. [Google Scholar] [CrossRef]
- Pal, M.; Subhedar, K.M. CNT yarn based solid state linear supercapacitor with multi-featured capabilities for wearable and implantable devices. Energy Storage Mater. 2023, 57, 136–170. [Google Scholar] [CrossRef]
- Yao, Y.; Huang, W.; Chen, J.; Liu, X.; Bai, L.; Chen, W.; Cheng, Y.; Ping, J.; Marks, T.J.; Facchetti, A. Flexible and Stretchable Organic Electrochemical Transistors for Physiological Sensing Devices. Adv. Mater. 2023, 35, 2209906. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Qin, H.; Xiao, X.; Liu, N.; Wang, S.; Li, J.; Shen, S.; Dai, S.; Sun, M.; Li, P.; et al. Robust hydrogel sensors for unsupervised learning enabled sign-to-verbal translation. InfoMat 2023, 5, e12419. [Google Scholar] [CrossRef]
- Zarepour, A.; Ahmadi, S.; Rabiee, N.; Zarrabi, A.; Iravani, S. Self-Healing MXene- and Graphene-Based Composites: Properties and Applications. Nano-Micro Lett. 2023, 15, 100. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ding, Q.; Wang, H.; Wu, Z.; Gui, X.; Li, C.; Hu, N.; Tao, K.; Wu, J. Engineering Smart Composite Hydrogels for Wearable Disease Monitoring. Nano-Micro Lett. 2023, 15, 1–45. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Cacucciolo, V.; Shea, H. Fiber pumps for wearable fluidic systems. Science 2023, 379, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Salauddin, M.; Rana, S.M.S.; Sharifuzzaman, M.; Song, H.S.; Reza, M.S.; Jeong, S.H.; Park, J.Y. Highly Electronegative V2CTx/Silicone Nanocomposite-Based Serpentine Triboelectric Nanogenerator for Wearable Self-Powered Sensors and Sign Language Interpretation. Adv. Energy Mater. 2023, 13, 2203812. [Google Scholar] [CrossRef]
- Min, J.; Tu, J.; Xu, C.; Lukas, H.; Shin, S.; Yang, Y.; Solomon, S.A.; Mukasa, D.; Gao, W. Skin-Interfaced Wearable Sweat Sensors for Precision Medicine. Chem. Rev. 2023, 123, 5049–5138. [Google Scholar] [CrossRef]
- Dou, W.; Yang, W.; Zhao, X.; Pan, Q. Hollow cobalt sulfide for highly efficient uranium adsorption from aqueous solutions. Inorg. Chem. Front. 2019, 6, 3230–3236. [Google Scholar] [CrossRef]
- Wei, J.; Xiao, P.; Chen, T. Water-Resistant Conductive gels toward Underwater Wearable Sensing. Adv. Mater. 2023, 35, 2211758. [Google Scholar] [CrossRef]
- Hu, H.; Huang, H.; Li, M.; Gao, X.; Yin, L.; Qi, R.; Wu, R.S.; Chen, X.; Ma, Y.; Shi, K.; et al. A wearable cardiac ultrasound imager. Nature 2023, 613, 667–675. [Google Scholar] [CrossRef]
- The Lancet Digital Health. Wearable health data privacy. Lancet Digit. Health 2023, 5, e174. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.B. A wearable sensor to measure troponin I levels. Nat. Rev. Cardiol. 2023, 20, 286. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, S.H.; Heo, J.S.; Kwak, J.Y.; Park, C.W.; Kim, I.; Lee, M.; Park, H.; Kim, Y.; Lee, S.J.; et al. Heterogeneous Structure Omnidirectional Strain Sensor Arrays with Cognitively Learned Neural Networks. Adv. Mater. 2023, 35, 2208184. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Song, Q.; Liu, K.; Liu, H.; Pan, J.; Liu, W.; Dai, L.; Zhang, M.; Wang, Y.; Si, C.; et al. Nanocellulose-Assisted Construction of Multifunctional MXene-Based Aerogels with Engineering Biomimetic Texture for Pressure Sensor and Compressible Electrode. Nano-Micro Lett. 2023, 15, 98. [Google Scholar] [CrossRef] [PubMed]
- Bathaei, M.J.; Singh, R.; Mirzajani, H.; Istif, E.; Akhtar, M.J.; Abbasiasl, T.; Beker, L. Photolithography-Based Microfabrication of Biodegradable Flexible and Stretchable Sensors. Adv. Mater. 2023, 35, 2207081. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Kwak, J.W.; Han, M.; Rogers, J.A. Mechanically Active Materials and Devices for Bio-Interfaced Pressure Sensors—A Review. Adv. Mater. 2022, 2205609. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-Y.; Moon, S.E.; Kim, J.H.; Kang, S.M. Ultrasensitive and Highly Stretchable Multiple-Crosslinked Ionic Hydrogel Sensors with Long-Term Stability. Nano-Micro Lett. 2023, 15, 51. [Google Scholar] [CrossRef]
- Feng, Z.; Hao, Y.; Qin, J.; Zhong, S.; Bi, K.; Zhao, Y.; Yin, L.; Pei, J.; Dang, Z. Ultrasmall barium titanate nanoparticles modulated stretchable dielectric elastomer sensors with large deformability and high sensitivity. InfoMat 2023, 5, e12413. [Google Scholar] [CrossRef]
- Su, Q.; Zou, Q.; Li, Y.; Chen, Y.; Teng, S.-Y.; Kelleher, J.T.; Nith, R.; Cheng, P.; Li, N.; Liu, W.; et al. A stretchable and strain-unperturbed pressure sensor for motion interference–free tactile monitoring on skins. Sci. Adv. 2021, 7, eabi4563. [Google Scholar] [CrossRef]
- Chen, M.; Wan, H.; Hu, Y.; Zhao, F.; An, X.; Lu, A. Rationally designed cellulose hydrogel for an ultrasensitive pressure sensor. Mater. Horiz. 2023, 10, 4510–4520. [Google Scholar] [CrossRef]
- Babamiri, B.; Bahari, D.; Salimi, A. Highly sensitive bioaffinity electrochemiluminescence sensors: Recent advances and future directions. Biosens. Bioelectron. 2019, 142, 111530. [Google Scholar] [CrossRef] [PubMed]
- Aykaç, A.; Gergeroglu, H.; Beşli, B.; Akkaş, E.; Yavaş, A.; Güler, S.; Güneş, F.; Erol, M. An Overview on Recent Progress of Metal Oxide/Graphene/CNTs-Based Nanobiosensors. Nanoscale Res. Lett. 2021, 16, 65. [Google Scholar] [CrossRef] [PubMed]
- Talikowska, M.; Fu, X.; Lisak, G. Application of conducting polymers to wound care and skin tissue engineering: A review. Biosens. Bioelectron. 2019, 135, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Vahdatiyekta, P.; Zniber, M.; Bobacka, J.; Huynh, T.-P. A review on conjugated polymer-based electronic tongues. Anal. Chim. Acta 2022, 1221, 340114. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Deng, N.; Zhao, H.; Wang, X.; Wei, L.; Wang, M.; Cheng, B.; Kang, W. A review on electronically conducting polymers for lithium-sulfur battery and lithium-selenium battery: Progress and prospects. J. Energy Chem. 2021, 58, 523–556. [Google Scholar] [CrossRef]
- Cui, M.; Che, Z.; Gong, Y.; Li, T.; Hu, W.; Wang, S. A graphdiyne-based protein molecularly imprinted biosensor for highly sensitive human C-reactive protein detection in human serum. Chem. Eng. J. 2022, 431, 133455. [Google Scholar] [CrossRef]
- Torres-García, R.; Flores-Estrada, J.; Cauich-Rodríguez, J.V.; Flores-Reyes, M.; Flores-Merino, M.V. Design of a polyacrylamide and gelatin hydrogel as a synthetic extracellular matrix. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 266–277. [Google Scholar] [CrossRef]
- Song, J.; Liu, H.; Zhao, Z.; Lin, P.; Yan, F. Flexible Organic Transistors for Biosensing: Devices and Applications. Adv. Mater. 2023, 2300034. [Google Scholar] [CrossRef]
- Li, J.; Wu, X.; Su, Y. An Overstretch Strategy to Double the Designed Elastic Stretchability of Stretchable Electronics. Adv. Mater. 2023, 35, 2300340. [Google Scholar] [CrossRef]
- Dai, Y.; Dai, S.; Li, N.; Li, Y.; Moser, M.; Strzalka, J.; Prominski, A.; Liu, Y.; Zhang, Q.; Li, S.; et al. Stretchable Redox-Active Semiconducting Polymers for High-Performance Organic Electrochemical Transistors. Adv. Mater. 2022, 34, 2201178. [Google Scholar] [CrossRef]
- Li, N.; Li, Y.; Cheng, Z.; Liu, Y.; Dai, Y.; Kang, S.; Li, S.; Shan, N.; Wai, S.; Ziaja, A.; et al. Bioadhesive polymer semiconductors and transistors for intimate biointerfaces. Science 2023, 381, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Qing, X.; Chen, H.; Zeng, F.; Jia, K.; Shu, Q.; Wu, J.; Xu, H.; Lei, W.; Liu, D.; Wang, X.; et al. All-Fiber Integrated Thermoelectrically Powered Physiological Monitoring Biosensor. Adv. Fiber Mater. 2023, 5, 1025–1036. [Google Scholar] [CrossRef]
- Mondal, R.; Hasan, A.M.; Baik, J.M.; Yang, Y. Advanced pyroelectric materials for energy harvesting and sensing applications. Mater. Today 2023, 66, 273–301. [Google Scholar] [CrossRef]
- Zhai, H.; Ding, S.; Chen, X.; Wu, Y.; Wang, Z.L. Advances in solid–solid contacting triboelectric nanogenerator for ocean energy harvesting. Mater. Today 2023, 65, 166–188. [Google Scholar] [CrossRef]
- Deng, Z.; Dapino, M.J. Review of magnetostrictive vibration energy harvesters. Smart Mater. Struct. 2017, 26, 103001. [Google Scholar] [CrossRef]
- Fratzl, P. Introduction: Sustainable Materials. Chem. Rev. 2023, 123, 1841–1842. [Google Scholar] [CrossRef]
- Zhi, C.; Shi, S.; Zhang, S.; Si, Y.; Yang, J.; Meng, S.; Fei, B.; Hu, J. Bioinspired All-Fibrous Directional Moisture-Wicking Electronic Skins for Biomechanical Energy Harvesting and All-Range Health Sensing. Nano-Micro Lett. 2023, 15, 60. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, Z.; Liu, Z.; Hu, Z. An Emerging Energy Technology: Self-Uninterrupted Electricity Power Harvesting from the Sun and Cold Space. Adv. Energy Mater. 2023, 13, 2300260. [Google Scholar] [CrossRef]
- Oh, I.H.; Park, E.; Chang, S.T.; Lim, S. Foldable RF Energy Harvesting System Based on Vertically Layered Metal Electrodes within a Single Sheet of Paper. Adv. Mater. 2023, 35, 2300197. [Google Scholar] [CrossRef]
- Dong, S.; Bu, T.; Wang, Z.; Feng, Y.; Liu, G.; Zeng, J.; Wang, Z.; Cao, J.; Zhang, Z.; Liu, F.; et al. Freestanding-Mode Tribovoltaic Nanogenerator for Harvesting Sliding and Rotational Mechanical Energy. Adv. Energy Mater. 2023, 13, 2300079. [Google Scholar] [CrossRef]
- Tan, J.; Wang, X.; Chu, W.; Fang, S.; Zheng, C.; Xue, M.; Wang, X.; Hu, T.; Guo, W. Harvesting Energy from Atmospheric Water: Grand Challenges in Continuous Electricity Generation. Adv. Mater. 2023, 2211165. [Google Scholar] [CrossRef]
- Shao, Y.; Luo, B.; Liu, T.; Cai, C.; Meng, X.; Wang, S.; Nie, S. Harvesting energy from extreme environmental conditions with cellulosic triboelectric materials. Mater. Today 2023, 66, 348–370. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, X.; Wang, Y.; Tian, C.; Wu, D.; Ning, N.; Tian, M. High Energy Harvesting Performances Silicone Elastomer via Filling Soft Dielectric with Stretching Deformability. Adv. Mater. 2023, 35, 2300246. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Yang, B.; Lan, S.; Liu, Y.; Liu, Y.; Nan, C.-W.; Lin, Y.-H. High-Entropy-Nanofibers Enhanced Polymer Nanocomposites for High-Performance Energy Storage. Adv. Energy Mater. 2023, 13, 2203925. [Google Scholar] [CrossRef]
- Zhang, J.; Zhuang, J.; Lei, L.; Hou, Y. Rapid preparation of a self-adhesive PAA ionic hydrogel using lignin sulfonate–Al3+ composite systems for flexible moisture-electric generators. J. Mater. Chem. A 2023, 11, 3546–3555. [Google Scholar] [CrossRef]
- Bian, J.; Wang, N.; Ma, J.; Jie, Y.; Zou, J.; Cao, X. Stretchable 3D polymer for simultaneously mechanical energy harvesting and biomimetic force sensing. Nano Energy 2018, 47, 442–450. [Google Scholar] [CrossRef]
- Guan, S.; Wang, J.; Yang, Y.; Zhu, X.; Zhou, J.; Ye, D.; Chen, R.; Dai, H.; Liao, Q. Highly Stretchable and Flexible Electrospinning-Based Biofuel Cell for Implantable Electronic. Adv. Funct. Mater. 2023, 33, 2303134. [Google Scholar] [CrossRef]
- Ohayon, D.; Nikiforidis, G.; Savva, A.; Giugni, A.; Wustoni, S.; Palanisamy, T.; Chen, X.; Maria, I.P.; Di Fabrizio, E.; Costa, P.M.F.J.; et al. Biofuel powered glucose detection in bodily fluids with an n-type conjugated polymer. Nat. Mater. 2020, 19, 456–463. [Google Scholar] [CrossRef]
- Luo, J.; Gao, W.; Wang, Z.L. The Triboelectric Nanogenerator as an Innovative Technology toward Intelligent Sports. Adv. Mater. 2021, 33, 2004178. [Google Scholar] [CrossRef]
- Dong, K.; Wu, Z.; Deng, J.; Wang, A.C.; Zou, H.; Chen, C.; Hu, D.; Gu, B.; Sun, B.; Wang, Z.L. A Stretchable Yarn Embedded Triboelectric Nanogenerator as Electronic Skin for Biomechanical Energy Harvesting and Multifunctional Pressure Sensing. Adv. Mater. 2018, 30, 1804944. [Google Scholar] [CrossRef]
- Lai, Y.-C.; Deng, J.; Liu, R.; Hsiao, Y.-C.; Zhang, S.L.; Peng, W.; Wu, H.-M.; Wang, X.; Wang, Z.L. Actively Perceiving and Responsive Soft Robots Enabled by Self-Powered, Highly Extensible, and Highly Sensitive Triboelectric Proximity- and Pressure-Sensing Skins. Adv. Mater. 2018, 30, 1801114. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, W.; Wang, S.; Yang, R. E-Polymers: Applications in Biological Interfaces and Organisms. Nanoenergy Adv. 2024, 4, 1-24. https://doi.org/10.3390/nanoenergyadv4010001
Dou W, Wang S, Yang R. E-Polymers: Applications in Biological Interfaces and Organisms. Nanoenergy Advances. 2024; 4(1):1-24. https://doi.org/10.3390/nanoenergyadv4010001
Chicago/Turabian StyleDou, Weixin, Sihong Wang, and Rusen Yang. 2024. "E-Polymers: Applications in Biological Interfaces and Organisms" Nanoenergy Advances 4, no. 1: 1-24. https://doi.org/10.3390/nanoenergyadv4010001