Polysaccharide Based Implantable Drug Delivery: Development Strategies, Regulatory Requirements, and Future Perspectives
Abstract
:1. Introduction
Classification of Implantable Drug Delivery Devices
2. Strategies to Employ Polysaccharides in Implant Formulation
3. Polysaccharide Based Polymers
3.1. Starch
3.2. Cellulose
3.3. Alginate
3.4. Chitosan
3.5. Pullulan
3.6. Carrageenan
3.7. Dextran
3.8. Hyaluronic Acid (HA)
3.9. Agar
3.10. Pectin
3.11. Gellan Gum
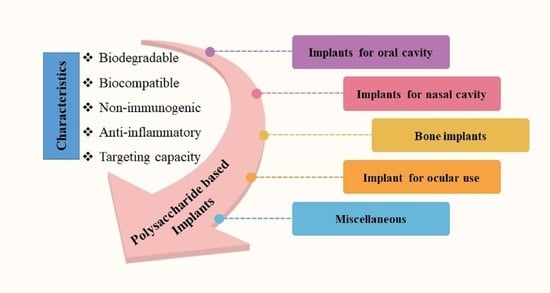
4. Biomedical Applications of Polysaccharide-Based Implantable Devices
4.1. Implants for Oral Cavity
4.2. Implants for Nasal Cavity
4.3. Bone Implants
4.4. Implant for Ocular Use
4.5. Implants for Antiviral Therapy
5. Regulatory Considerations for Implantable Device
5.1. Important Laboratory Testing Required for Approval
5.1.1. Material Characterization
5.1.2. Biocompatibility
5.1.3. Sterility
6. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dash, A.; Cudworth, G. Therapeutic applications of implantable drug delivery systems. J. Pharmacol. Toxicol. Methods 1998, 40, 1–12. [Google Scholar] [CrossRef]
- Stewart, S.A.; Domínguez-Robles, J.; Donnelly, R.F.; Larrañeta, E. Implantable Polymeric Drug Delivery Devices: Classification, Manufacture, Materials, and Clinical Applications. Polymers 2018, 10, 1379. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pillai, J. Implantable Drug Delivery Systems: An Overview. In Nanostructures for the Engineering of Cells, Tissues and Organs From Design to Applications; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 473–511. [Google Scholar]
- McDonald, M.; D’Aversa, G.; Perry, H.D.; Wittpenn, J.R.; Donnenfeld, E.D.; Nelinson, D.S. Hydroxypropyl cellulose ophthalmic inserts (lacrisert) reduce the signs and symptoms of dry eye syndrome and improve patient quality of life. Trans. Am. Ophthalmol. Soc. 2009, 107, 214–221. [Google Scholar]
- Retisert. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/021737s007lbl.pdf (accessed on 29 August 2022).
- Yadav, P.; Yadav, H.; Shah, V.G.; Shah, G.; Dhaka, G. Biomedical Biopolymers, Their Origin and Evolution in Biomedical Sciences: A Systematic Review. J. Clin. Diagn. Res. 2015, 9, ZE21-5. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, R.; Fernandes, M.; Fangueiro, R. Biopolymers in Medical Implants: A Brief Review. Procedia Eng. 2017, 200, 236–243. [Google Scholar] [CrossRef]
- Prasher, P.; Sharma, M.; Mehta, M.; Satija, S.; Aljabali, A.A.; Tambuwala, M.M.; Anand, K.; Sharma, N.; Dureja, H.; Jha, N.K.; et al. Current-status and applications of polysaccharides in drug delivery systems. Colloids Interface Sci. Commun. 2021, 42, 100418. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, H.; Fu, Q.; Xie, X.; Song, Y.; Xu, M.; Li, J. 3D-printed polycaprolactone-chitosan based drug delivery implants for personalized administration. Mater. Des. 2022, 214, 110394. [Google Scholar] [CrossRef]
- López-Valverde, N.; López-Valverde, A.; Cortés, M.P.; Rodríguez, C.; De Sousa, B.M.; Aragoneses, J.M. Bone Quantification Around Chitosan-Coated Titanium Dental Implants: A Preliminary Study by Micro-CT Analysis in Jaw of a Canine Model. Front. Bioeng. Biotechnol. 2022, 10, 858786. [Google Scholar] [CrossRef]
- Nganga, S.; Travan, A.; Marsich, E.; Donati, I.; Söderling, E.; Moritz, N.; Paoletti, S.; Vallittu, P.K. In vitro antimicrobial properties of silver–polysaccharide coatings on porous fiber-reinforced composites for bone implants. J. Mater. Sci. Mater. Electron. 2013, 24, 2775–2785. [Google Scholar] [CrossRef]
- AlNufaiy, B.M.; Lambarte, R.N.A.; Al-Hamdan, K.S. The Osteogenetic Potential of Chitosan Coated Implant: An In Vitro Study. J. Stem Cells Regen. Med. 2020, 16, 44–49. [Google Scholar] [CrossRef]
- Kim, H.; Tator, C.H.; Shoichet, M.S. Chitosan implants in the rat spinal cord: Biocompatibility and biodegradation. J. Biomed. Mater. Res. Part A 2011, 97A, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Nawrotek, K.; Makiewicz, M.; Zawadzki, D. Fabrication and Characterisation of Polycaprolactone/Chitosan—Hydroxyapatite Hybrid Implants for Peripheral Nerve Regeneration. Polymers 2021, 13, 775. [Google Scholar] [CrossRef] [PubMed]
- Norowski, P.A.; Courtney, H.S.; Babu, J.; Haggard, W.O.; Bumgardner, J.D. Chitosan Coatings Deliver Antimicrobials From Titanium Implants: A Preliminary Study. Implant Dent. 2011, 20, 56–67. [Google Scholar] [CrossRef]
- Campelo, C.S.; Chevallier, P.; Vaz, J.M.; Vieira, R.S.; Mantovani, D. Sulfonated chitosan and dopamine based coatings for metallic implants in contact with blood. Mater. Sci. Eng. C 2017, 72, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Aho, J.; Halme, A.; Boetker, J.; Water, J.J.; Bohr, A.; Sandler, N.; Rantanen, J.; Baldursdottir, S. The effect of HPMC and MC as pore formers on the rheology of the implant microenvironment and the drug release in vitro. Carbohydr. Polym. 2017, 177, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Zhao, X.; Liu, S.; Chen, M.; Liu, B.; Ge, J.; Jia, Y.-G.; Ren, L. Collagen–Hydroxypropyl Methylcellulose Membranes for Corneal Regeneration. ACS Omega 2018, 3, 1269–1275. [Google Scholar] [CrossRef]
- Addition of Hydroxypropyl Methylcellulose to Hydroxyapatite-Chitosan Composite as An Injectable Bone Substitute. Available online: https://aip.scitation.org/doi/pdf/10.1063/5.0004043 (accessed on 28 July 2022).
- Sheskey, P.J.; Hancock, B.C.; Moss, G.P.; Goldfarb, D.J. Handbook of Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press: London, UK, 2009; pp. 1–917. [Google Scholar]
- Kunle, O.O. Starch Source and Its Impact on Pharmaceutical Applications. In Chemical Properties of Starch; Emeje, M., Ed.; IntechOpen: London, UK, 2020. [Google Scholar]
- Masina, N.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Govender, M.; Indermun, S.; Pillay, V. A review of the chemical modification techniques of starch. Carbohydr. Polym. 2017, 157, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ramsden, L.; Corke, H. Physical properties and enzymatic digestibility of hydroxypropylated ae, wx, and normal maize starch. Carbohydr. Polym. 1999, 40, 175–182. [Google Scholar] [CrossRef]
- Adetunji, A.O. Chemically Modified Starches as Excipients in Pharmaceutical Dosage Forms. In Chemical Properties of Starch; Emeje, M., Ed.; IntechOpen: London, UK, 2020. [Google Scholar]
- Nawaz, H.; Waheed, R.; Nawaz, M.; Shahwar, D. Physical and Chemical Modifications in Starch Structure and Reactivity. In Chemical Properties of Starch; Emeje, M., Ed.; IntechOpen: London, UK, 2020. [Google Scholar]
- Lemos, P.V.F.; Marcelino, H.R.; Cardoso, L.G.; de Souza, C.O.; Druzian, J.I. Starch chemical modifications applied to drug delivery systems: From fundamentals to FDA-approved raw materials. Int. J. Biol. Macromol. 2021, 184, 218–234. [Google Scholar] [CrossRef]
- Hsieh, Y.-C.; Yano, H.; Nogi, M.; Eichhorn, S.J. An estimation of the Young’s modulus of bacterial cellulose filaments. Cellulose 2008, 15, 507–513. [Google Scholar] [CrossRef]
- Nakagaito, A.N.; Iwamoto, S.; Yano, H. Bacterial cellulose: The ultimate nano-scalar cellulose morphology for the production of high-strength composites. Appl. Phys. A Mater. Sci. Process. 2005, 80, 93–97. [Google Scholar] [CrossRef]
- Brown, R.M.; Willison, J.H.; Richardson, C.L. Cellulose biosynthesis in Acetobacter xylinum: Visualization of the site of synthesis and direct measurement of the in vivo process. Proc. Natl. Acad. Sci. USA 1976, 73, 4565–4569. [Google Scholar] [CrossRef]
- Trovatti, E.; Oliveira, L.; Freire, C.S.; Silvestre, A.; Neto, C.; Pinto, J.J.C.; Gandini, A. Novel bacterial cellulose–acrylic resin nanocomposites. Compos. Sci. Technol. 2010, 70, 1148–1153. [Google Scholar] [CrossRef]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose Nanofibers Prepared by TEMPO-Mediated Oxidation of Native Cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Zimnitsky, D.S.; Yurkshtovich, T.L.; Bychkovsky, P.M. Synthesis and Characterisation of Oxidized Cellulose. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 4785–4791. [Google Scholar] [CrossRef]
- Müller, F.A.; Müller, L.; Hofmann, I.; Greil, P.; Wenzel, M.M.; Staudenmaier, R. Cellulose-based scaffold materials for cartilage tissue engineering. Biomaterials 2006, 27, 3955–3963. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, M.G.; Sandmann, G.; Schubert, T.; Klein, D. Effect of oxidized regenerated cellulose/collagen matrix on dermal and epidermal healing and growth factors in an acute wound. Wound Repair Regen. 2005, 13, 324–331. [Google Scholar] [CrossRef]
- Bassetto, F.; Vindigni, V.; Scarpa, C.; Botti, C.; Botti, G. Use of Oxidized Regenerated Cellulose to Stop Bleeding After a Facelift Procedure. Aesthetic Plast. Surg. 2008, 32, 807–809. [Google Scholar] [CrossRef]
- Stanford, E.C.C. On algin: A new substance obtained from some of the commoner species of marine algae. Am. J. Pharm. 1883, 16, 6323–6324. [Google Scholar] [CrossRef]
- Wasikiewicz, J.; Yoshii, F.; Nagasawa, N.; Wach, R.; Mitomo, H. Degradation of chitosan and sodium alginate by gamma radiation, sonochemical and ultraviolet methods. Radiat. Phys. Chem. 2005, 73, 287–295. [Google Scholar] [CrossRef]
- Remminghorst, U.; Rehm, B.H.A. Bacterial alginates: From biosynthesis to applications. Biotechnol. Lett. 2006, 28, 1701–1712. [Google Scholar] [CrossRef]
- Smidsrød, O.; Haug, A.; Larsen, B. The Influence of pH on the Rate of Hydrolysis of Acidic Polysaccharides. Acta Chem. Scand. 1966, 20, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2016, 25, 854–866. [Google Scholar] [CrossRef]
- Huq, T.; Khan, A.; Brown, D.; Dhayagude, N.; He, Z.; Ni, Y. Sources, production and commercial applications of fungal chitosan: A review. J. Bioresour. Bioprod. 2022, 7, 85–98. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.-T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as Antimicrobial Agent: Applications and Mode of Action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Shariatinia, Z. Pharmaceutical applications of chitosan. Adv. Colloid Interface Sci. 2018, 263, 131–194. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156. [Google Scholar] [CrossRef]
- Ojeda-Hernández, D.D.; Canales-Aguirre, A.A.; Matias-Guiu, J.; Gomez-Pinedo, U.; Mateos-Díaz, J.C. Potential of Chitosan and Its Derivatives for Biomedical Applications in the Central Nervous System. Front. Bioeng. Biotechnol. 2020, 8, 389. [Google Scholar] [CrossRef]
- Wang, W.; Xue, C.; Mao, X. Chitosan: Structural Modification, Biological Activity and Application. Int. J. Biol. Macromol. 2020, 164, 4532–4546. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Rana, V.; Kennedy, J.F. Recent insights on applications of pullulan in tissue engineering. Carbohydr. Polym. 2016, 153, 455–462. [Google Scholar] [CrossRef]
- Singh, R.S.; Saini, G.K. Biosynthesis of Pullulan and Its Applications in Food and Pharmaceutical Industry. Microorg. Sustain. Agric. Biotechnol. 2012, 9789400722, 509–553. [Google Scholar]
- Cheng, K.-C.; Demirci, A.; Catchmark, J.M. Pullulan: Biosynthesis, production, and applications. Appl. Microbiol. Biotechnol. 2011, 92, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Patil, R.; Dubey, S.K.; Bahadur, P. Derivatization approaches and applications of pullulan. Adv. Colloid Interface Sci. 2019, 269, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Jin, Z.; Kim, J.M.; Tong, Q.; Chen, H. Graft copolymerization of methyl acrylate onto pullulan using ceric ammonium nitrate as initiator. Carbohydr. Polym. 2009, 76, 129–132. [Google Scholar] [CrossRef]
- Glinel, K.; Sauvage, J.P.; Oulyadi, H.; Huguet, J. Determination of substituents distribution in carboxymethylpullulans by NMR spectroscopy. Carbohydr. Res. 2000, 328, 343–354. [Google Scholar] [CrossRef]
- Pacheco-Quito, E.-M.; Ruiz-Caro, R.; Veiga, M.-D. Carrageenan: Drug Delivery Systems and Other Biomedical Applications. Mar. Drugs 2020, 18, 583. [Google Scholar] [CrossRef]
- De Ruiter, G.A.; Rudolph, B. Carrageenan biotechnology. Trends Food Sci. Technol. 1997, 8, 389–395. [Google Scholar] [CrossRef]
- Mihaila, S.M.; Gaharwar, A.K.; Reis, R.L.; Marques, A.P.; Gomes, M.E.; Khademhosseini, A. Photocrosslinkable Kappa -Carrageenan Hydrogels for Tissue Engineering Applications. Adv. Health Mater. 2012, 2, 895–907. [Google Scholar] [CrossRef]
- Mokhtari, H.; Tavakoli, S.; Safarpour, F.; Kharaziha, M.; Bakhsheshi-Rad, H.; Ramakrishna, S.; Berto, F. Recent Advances in Chemically-Modified and Hybrid Carrageenan-Based Platforms for Drug Delivery, Wound Healing, and Tissue Engineering. Polymers 2021, 13, 1744. [Google Scholar] [CrossRef]
- Liu, J.; Zhan, X.; Wan, J.; Wang, Y.; Wang, C. Review for carrageenan-based pharmaceutical biomaterials: Favourable physical features versus adverse biological effects. Carbohydr. Polym. 2014, 121, 27–36. [Google Scholar] [CrossRef]
- Naessens, M.; Cerdobbel, A.; Soetaert, W.; Vandamme, E.J. Leuconostoc dextransucrase and dextran: Production, properties and applications. J. Chem. Technol. Biotechnol. 2005, 80, 845–860. [Google Scholar] [CrossRef]
- Sarwat, F.; Qader, S.A.U.; Aman, A.; Ahmed, N. Production & Characterisation of a Unique Dextran from an Indigenous Leuconostoc Mesenteroides CMGInt. J. Biol. Sci. 2008, 4, 379–386. [Google Scholar]
- Díaz-Montes, E. Dextran: Sources, Structures, and Properties. Polysaccharides 2021, 2, 554–565. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Saeb, M.R.; Jafari, S.H.; Mozafari, M. Application of Compatibilized Polymer Blends in Biomedical Fields. In Compatibilization of Polymer Blends; Elsevier: Amsterdam, The Netherlands, 2020; pp. 511–537. [Google Scholar] [CrossRef]
- Hu, Q.; Lu, Y.; Luo, Y. Recent advances in dextran-based drug delivery systems: From fabrication strategies to applications. Carbohydr. Polym. 2021, 264, 117999. [Google Scholar] [CrossRef] [PubMed]
- Tirtaatmadja, V.; Dunstan, D.E.; Boger, D.V. Rheology of dextran solutions. J. Non-Newtonian Fluid Mech. 2001, 97, 295–301. [Google Scholar] [CrossRef]
- Zarour, K.; Llamas, M.G.; Prieto, A.; Rúas-Madiedo, P.; Dueñas, M.T.; de Palencia, P.F.; Aznar, R.; Kihal, M.; López, P. Rheology and bioactivity of high molecular weight dextrans synthesised by lactic acid bacteria. Carbohydr. Polym. 2017, 174, 646–657. [Google Scholar] [CrossRef]
- Kobayashi, M.; Funane, K. Condensation of Dextran-dialdehyde with Amino Acids under Nonreductive Conditions. Biosci. Biotechnol. Biochem. 1993, 57, 881–883. [Google Scholar] [CrossRef]
- Simoni, R.D.; Hill, R.L.; Vaughan, M.; Hascall, V. The Discovery of Hyaluronan by Karl Meyer. J. Biol. Chem. 2002, 277, e1–e2. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic acid (hyaluronan): A review. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Dermato-Endocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Li, J.; Du, G.; Chen, J. Microbial production of hyaluronic acid: Current state, challenges, and perspectives. Microb. Cell Factories 2011, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Maleki, A.; Kjøniksen, A.-L.; Nyström, B. Effect of pH on the Behavior of Hyaluronic Acid in Dilute and Semidilute Aqueous Solutions. Macromol. Symp. 2008, 274, 131–140. [Google Scholar] [CrossRef]
- Pisárčik, M.; Bakoš, D.; Čeppan, M. Non-Newtonian properties of hyaluronic acid aqueous solution. Colloids Surfaces A Physicochem. Eng. Asp. 1995, 97, 197–202. [Google Scholar] [CrossRef]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Phillips, G.O.; Williams, P.A. Handbook of Hydrocolloids, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 1–924. [Google Scholar] [CrossRef]
- Training Manual on Gracilaria Culture and Seaweed Processing in China. Available online: https://agris.fao.org/agris-search/search.do?recordID=XF9214331 (accessed on 28 July 2022).
- BeMiller, J.N. An Introduction to Pectins: Structure and Properties. In Chemistry and Function of Pectins; Fishman, M.L., Jen, J.J., Eds.; American Chemical Society: Washington, DC, USA, 1986; pp. 2–12. [Google Scholar]
- Willats, W.G.; Knox, J.P.; Mikkelsen, J.D. Pectin: New insights into an old polymer are starting to gel. Trends Food Sci. Technol. 2006, 17, 97–104. [Google Scholar] [CrossRef]
- Voragen, A.G.J.; Coenen, G.-J.; Verhoef, R.P.; Schols, H.A. Pectin, a versatile polysaccharide present in plant cell walls. Struct. Chem. 2009, 20, 263–275. [Google Scholar] [CrossRef]
- Zia, K.M.; Tabasum, S.; Khan, M.F.; Akram, N.; Akhter, N.; Noreen, A.; Zuber, M. Recent trends on gellan gum blends with natural and synthetic polymers: A review. Int. J. Biol. Macromol. 2017, 109, 1068–1087. [Google Scholar] [CrossRef]
- Coviello, T.; Matricardi, P.; Marianecci, C.; Alhaique, F. Polysaccharide hydrogels for modified release formulations. J. Control. Release 2007, 119, 5–24. [Google Scholar] [CrossRef]
- Silva, S.; Fernandes, E.; Pina, S.; Silva-Correia, J.; Vieira, S.; Oliveira, J.; Reis, R. Polymers of Biological Origin. In Comprehensive Biomaterials; Ducheyne, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 228–252. [Google Scholar] [CrossRef]
- Rinaudo, M.; Milas, M. Gellan Gum, a Bacterial Gelling Polymer. Dev. Food Sci. 2000, 41, 239–263. [Google Scholar] [CrossRef]
- Jiang, T.; Duan, Q.; Zhu, J.; Liu, H.; Yu, L. Starch-based biodegradable materials: Challenges and opportunities. Adv. Ind. Eng. Polym. Res. 2019, 3, 8–18. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, M.; Shen, J.; He, Z.; Fatehi, P.; Ni, Y. Applications of Cellulose-based Materials in Sustained Drug Delivery Systems. Curr. Med. Chem. 2019, 26, 2485–2501. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Kaur, N.; Rana, V.; Kennedy, J.F. Pullulan: A novel molecule for biomedical applications. Carbohydr. Polym. 2017, 171, 102–121. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Saeb, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-based biomaterials for tissue engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef]
- Freitas, C.; Coimbra, J.; Souza, V.; Sousa, R. Structure and Applications of Pectin in Food, Biomedical, and Pharmaceutical Industry: A Review. Coatings 2021, 11, 922. [Google Scholar] [CrossRef]
- Tripodo, G.; Trapani, A.; Torre, M.L.; Giammona, G.; Trapani, G.; Mandracchia, D. Hyaluronic acid and its derivatives in drug delivery and imaging: Recent advances and challenges. Eur. J. Pharm. Biopharm. 2015, 97, 400–416. [Google Scholar] [CrossRef]
- Agibayeva, L.E.; Kaldybekov, D.B.; Porfiryeva, N.N.; Garipova, V.R.; Mangazbayeva, R.A.; Moustafine, R.I.; Semina, I.I.; Mun, G.A.; Kudaibergenov, S.E.; Khutoryanskiy, V.V. Gellan gum and its methacrylated derivatives as in situ gelling mucoadhesive formulations of pilocarpine: In vitro and in vivo studies. Int. J. Pharm. 2020, 577, 119093. [Google Scholar] [CrossRef]
- Agarwal, C.; Csóka, L. Surface-Modified Cellulose in Biomedical Engineering. In Materials for Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 215–261. [Google Scholar] [CrossRef]
- Zennifer, A.; Senthilvelan, P.; Sethuraman, S.; Sundaramurthi, D. Key advances of carboxymethyl cellulose in tissue engineering & 3D bioprinting applications. Carbohydr. Polym. 2020, 256, 117561. [Google Scholar] [CrossRef]
- Ciecholewska-Juśko, D.; Żywicka, A.; Junka, A.; Drozd, R.; Sobolewski, P.; Migdał, P.; Kowalska, U.; Toporkiewicz, M.; Fijałkowski, K. Superabsorbent crosslinked bacterial cellulose biomaterials for chronic wound dressings. Carbohydr. Polym. 2020, 253, 117247. [Google Scholar] [CrossRef]
- Chen, J.; Liu, W.; Liu, C.-M.; Li, T.; Liang, R.-H.; Luo, S.-J. Pectin Modifications: A Review. Crit. Rev. Food Sci. Nutr. 2013, 55, 1684–1698. [Google Scholar] [CrossRef]
- Deo, P.N.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122–128. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/oral-health (accessed on 14 December 2021).
- Baranov, N.; Popa, M.; Atanase, L.; Ichim, D. Polysaccharide-Based Drug Delivery Systems for the Treatment of Periodontitis. Molecules 2021, 26, 2735. [Google Scholar] [CrossRef] [PubMed]
- Carolina Ramirez Hernandez, F. Use of Hyaluronic Acid in Osteointegration of Dental Implants. Sci. Arch. Dent. Sci. 2019, 2, 27–28. [Google Scholar]
- Cervino, G.; Meto, A.; Fiorillo, L.; Odorici, A.; Meto, A.; D’Amico, C.; Oteri, G.; Cicciù, M. Surface Treatment of the Dental Implant with Hyaluronic Acid: An Overview of Recent Data. Int. J. Environ. Res. Public Health 2021, 18, 4670. [Google Scholar] [CrossRef]
- López-Valverde, N.; López-Valverde, A.; Ramírez, J. Systematic Review of Effectiveness of Chitosan as a Biofunctionalizer of Titanium Implants. Biology 2021, 10, 102. [Google Scholar] [CrossRef]
- Lan, S.-F.; Kehinde, T.; Zhang, X.; Khajotia, S.; Schmidtke, D.W.; Starly, B. Controlled release of metronidazole from composite poly-ε-caprolactone/alginate (PCL/alginate) rings for dental implants. Dent. Mater. 2013, 29, 656–665. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, H.H.; Kim, S.H.; Hwang, S.H. Effectiveness of using a bioabsorbable implant (Latera) to treat nasal valve collapse in patients with nasal obstruction: Systemic review and meta-analysis. Int. Forum Allergy Rhinol. 2020, 10, 719–725. [Google Scholar] [CrossRef]
- Romo, T.; Pearson, J.M. Nasal Implants. Facial Plast. Surg. Clin. N. Am. 2008, 16, 123–132. [Google Scholar] [CrossRef]
- Sclafani, A.P.; Romo, T.; Iii, M. Biology and Chemistry of Facial Implants. Facial Plast. Surg. 2000, 16, 3–6. [Google Scholar] [CrossRef]
- Parikh, A.; Anand, U.; Ugwu, M.C.; Feridooni, T.; Massoud, E.; Agu, R.U. Drug-Eluting Nasal Implants: Formulation, Characterization, Clinical Applications and Challenges. Pharmaceutics 2014, 6, 249–267. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Park, J.; Heo, J.; Lee, S.-E.; Shin, J.-W.; Chang, M.; Hong, J. Polysaccharide-based superhydrophilic coatings with antibacterial and anti-inflammatory agent-delivering capabilities for ophthalmic applications. J. Ind. Eng. Chem. 2018, 68, 229–237. [Google Scholar] [CrossRef]
- Feng, Z.; Li, M.; Jin, X.; Zheng, Y.; Liu, J.; Zhao, L.; Wang, Y.; Li, H.; Zuo, D. Design and characterization of plasticized bacterial cellulose/waterborne polyurethane composite with antibacterial function for nasal stenting. Regen. Biomater. 2020, 7, 597–608. [Google Scholar] [CrossRef]
- Sang, S.; Ma, Z.; Cao, Y.; Shen, Z.; Duan, J.; Zhang, Y.; Wang, L.; An, Y.; Mao, X.; An, Y.; et al. BC enhanced photocurable hydrogel based on 3D bioprinting for nasal cartilage repair. Int. J. Polym. Mater. Polym. Biomater. 2022, 1–12. [Google Scholar] [CrossRef]
- Bleier, B.S.; Kofonow, J.M.; Hashmi, N.; Chennupati, S.K.; Cohen, N. Antibiotic Eluting Chitosan Glycerophosphate Implant in the Setting of Acute Bacterial Sinusitis: A Rabbit Model. Am. J. Rhinol. Allergy 2010, 24, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Spałek, J.; Ociepa, P.; Deptuła, P.; Piktel, E.; Daniluk, T.; Król, G.; Góźdź, S.; Bucki, R.; Okła, S. Biocompatible Materials in Otorhinolaryngology and Their Antibacterial Properties. Int. J. Mol. Sci. 2022, 23, 2575. [Google Scholar] [CrossRef]
- Oladapo, B.I.; Zahedi, S.A.; Ismail, S.O.; Olawade, D.B. Recent advances in biopolymeric composite materials: Future sustainability of bone-implant. Renew. Sustain. Energy Rev. 2021, 150, 111505. [Google Scholar] [CrossRef]
- Costa-Pinto, A.R.; Reis, R.L.; Neves, N.M. Scaffolds Based Bone Tissue Engineering: The Role of Chitosan. Tissue Eng. Part B Rev. 2011, 17, 331–347. [Google Scholar] [CrossRef]
- Frohbergh, M.E.; Katsman, A.; Botta, G.P.; Lazarovici, P.; Schauer, C.L.; Wegst, U.G.; Lelkes, P.I. Electrospun hydroxyapatite-containing chitosan nanofibers crosslinked with genipin for bone tissue engineering. Biomaterials 2012, 33, 9167–9178. [Google Scholar] [CrossRef]
- Lee, E.-J.; Kim, H.-E. Accelerated bony defect healing by chitosan/silica hybrid membrane with localized bone morphogenetic protein-2 delivery. Mater. Sci. Eng. C 2016, 59, 339–345. [Google Scholar] [CrossRef]
- Agarwal, T.; Kabiraj, P.; Narayana, G.H.; Kulanthaivel, S.; Kasiviswanathan, U.; Pal, K.; Giri, S.; Maiti, T.K.; Banerjee, I. Alginate Bead Based Hexagonal Close Packed 3D Implant for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2016, 8, 32132–32145. [Google Scholar] [CrossRef]
- Wu, M.; Wu, P.; Xiao, L.; Zhao, Y.; Yan, F.; Liu, X.; Xie, Y.; Zhang, C.; Chen, Y.; Cai, L. Biomimetic mineralization of novel hydroxyethyl cellulose/soy protein isolate scaffolds promote bone regeneration in vitro and in vivo. Int. J. Biol. Macromol. 2020, 162, 1627–1641. [Google Scholar] [CrossRef] [PubMed]
- Faria, J.; Dionísio, B.; Soares, Í.; Baptista, A.C.; Marques, A.; Gonçalves, L.; Bettencourt, A.; Baleizão, C.; Ferreira, I. Cellulose acetate fibres loaded with daptomycin for metal implant coatings. Carbohydr. Polym. 2021, 276, 118733. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.; Bose, S. 3D Printed Hydroxyapatite–Zn2+ Functionalized Starch Composite Bone Grafts for Orthopedic and Dental Applications. Mater. Des. 2022, 221, 110903. [Google Scholar] [CrossRef]
- Ghosh, S.; Viana, J.; Reis, R.; Mano, J. Bi-layered constructs based on poly(l-lactic acid) and starch for tissue engineering of osteochondral defects. Mater. Sci. Eng. C 2008, 28, 80–86. [Google Scholar] [CrossRef]
- Fricain, J.C.; Schlaubitz, S.; Le Visage, C.; Arnault, I.; Derkaoui, S.M.; Siadous, R.; Catros, S.; Lalande, C.; Bareille, R.; Renard, M.; et al. A nano-hydroxyapatite–Pullulan/dextran polysaccharide composite macroporous material for bone tissue engineering. Biomaterials 2013, 34, 2947–2959. [Google Scholar] [CrossRef]
- Martins, A.F.; Vlcek, J.; Wigmosta, T.; Hedayati, M.; Reynolds, M.M.; Popat, K.C.; Kipper, M.J. Chitosan/iota-carrageenan and chitosan/pectin polyelectrolyte multilayer scaffolds with antiadhesive and bactericidal properties. Appl. Surf. Sci. 2019, 502, 144282. [Google Scholar] [CrossRef]
- Marõ´a, M.; Alonso, J.; Sá Nchez, A.; Alonso, M.J.; Mara, M.; Sá, A. The Potential of Chitosan in Ocular Drug Delivery. J. Pharm. Pharmacol. 2010, 55, 1451–1463. [Google Scholar]
- Manna, S.; Augsburger, J.J.; Correa, Z.M.; Landero, J.A.; Banerjee, R.K. Development of Chitosan and Polylactic Acid Based Methotrexate Intravitreal Micro-Implants to Treat Primary Intraocular Lymphoma: An In Vitro Study. J. Biomech. Eng. 2014, 136, 021018. [Google Scholar] [CrossRef]
- Manna, S.; Donnell, A.M.; Kaval, N.; Al-Rjoub, M.F.; Augsburger, J.J.; Banerjee, R.K. Improved Design and Characterisation of PLGA/PLA-Coated Chitosan Based Micro-Implants for Controlled Release of Hydrophilic Drugs. Int. J. Pharm. 2018, 547, 122–132. [Google Scholar] [CrossRef]
- Drug Approval Package: Retisert (Fluocinolone Acetonide Intravireal Implant) NDA. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021737s000TOC.cfm (accessed on 28 July 2022).
- Jaffe, G.J.; Martin, D.; Callanan, D.; Pearson, P.A.; Levy, B.; Comstock, T. Fluocinolone Acetonide Implant (Retisert) for Noninfectious Posterior Uveitis: Thirty-Four–Week Results of a Multicenter Randomized Clinical Study. Ophthalmology 2006, 113, 1020–1027. [Google Scholar] [CrossRef]
- Lacrisert. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2002/18771s12lbl.pdf (accessed on 28 July 2022).
- Shenge, J.A.; Opayele, A.V. The Impact and Control of Emerging and Re-Emerging Viral Diseases in the Environment: An African Perspective. Curr. Microbiol. Res. Africa Sel. Appl. Sustain. Environ. Manag. 2020, 5, 185–202. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. HIV/AIDS. Available online: https://www.who.int/news-room/fact-sheets/detail/hiv-aids (accessed on 10 February 2022).
- Weld, E.D.; Flexner, C. Long-acting implants to treat and prevent HIV infection. Curr. Opin. HIV AIDS 2020, 15, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Maturavongsadit, P.; Shrivastava, R.; Sykesc, C.; Cottrell, M.L.; Montgomery, S.A.; Kashuba, A.D.M.; Benhabbour, S.R. Biodegradable Polymeric Solid Implants for Ultra-Long-Acting Delivery of Single or Multiple Antiretroviral Drugs. Int. J. Pharm. 2021, 605, 120844. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, I.C.P.; Campo, K.N.; Arns, C.W.; Gabriel, L.P.; Webster, T.J.; Lopes, É.S.N. From Bulk to Nanoparticles: An Overview of Antiviral Materials, Its Mechanisms, and Applications. Part. Part. Syst. Charact. 2021, 38, 2100044. [Google Scholar] [CrossRef]
- Otto, D.P.; de Villiers, M.M. Layer-by-Layer Nanocoating of Antiviral Polysaccharides on Surfaces to Prevent Coronavirus Infections. Molecules 2020, 25, 3415. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Yang, Z.; Chen, J.; Wang, D.; Zhang, Y. Recent advances in antiviral activities and potential mechanisms of sulfated polysaccharides. Carbohydr. Polym. 2021, 272, 118526. [Google Scholar] [CrossRef]
- FDA. Regulatory Controls. Available online: https://www.fda.gov/medical-devices/overview-device-regulation/regulatory-controls (accessed on 29 August 2022).
- FDA. How to Find and Effectively Use Predicate Devices. Available online: https://www.fda.gov/medical-devices/premarket-notification-510k/how-find-and-effectively-use-predicate-devices (accessed on 29 August 2022).
- Zhang, N.; Baume, A.; Payne, R.; Allickson, J. Regulatory Aspects: Regulation of Cell-Free Biomaterial Implants. In Situ Tissue Regeneration; Jin Lee, S., Yoo, J.J., Atala, A., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 383–403. [Google Scholar]
- Center for Devices and Radiological Health. Design Control Guidance Design Control Guidance for Medical Device Manufacturers. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/design-control-guidance-medical-device-manufacturers (accessed on 29 August 2022).
- Guidance for the Preparation of a Premarket Notification Application for a Surgical Mesh-Guidance for Industry and/or for FDA Reviewers/Staff and/or Compliance FDA. Available online: https://www.hhs.gov/guidance/document/guidance-preparation-premarket-notification-application-surgical-mesh-guidance-industry (accessed on 29 August 2022).
- Use of International Standard ISO 10993-1, “Biological Evaluation of Medical Devices-Part 1: Evaluation and Testing within a Risk Management Process” Guidance for Industry and Food and Drug Administration Staff. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/use-international-standard-iso-10993-1-biological-evaluation-medical-devices-part-1-evaluation-and (accessed on 28 July 2022).











| Name of the Marketed Ocular Inserts | Polysaccharides Used | Indication | Site of Implantation |
|---|---|---|---|
| LACRISERT® | Hydroxy propyl cellulose | Dry Eye disease (Keratoconjunctivitis Sicca) | Cul-de-Sac of the inferior eyelid |
| RETISERT™ | Microcrystalline cellulose | Chronic uveitis | Posterior region of the eye |
| Origin | Polysaccharides |
|---|---|
| Plant/algal | Starch (amylose/amylopectin), cellulose, agar, alginate, carrageenan, pectin, konjac, guar gum |
| Animal | Chitin/chitosan, hyaluronic acid |
| Bacterial | Xanthan, dextran, gellan, levan, curdlan, polygalactosamine |
| Fungal | Pullulan, elsinan, yeast glucans |
| Polysaccharide | Advantages | Disadvantages | References |
|---|---|---|---|
| Starch |
|
| [82] |
| Cellulose |
|
| [83] |
| Alginate |
|
| [84] |
| Chitosan |
|
| [42,43] |
| Pullulan |
|
| [85] |
| Carrageenan |
|
| [57] |
| Dextran |
|
| [62] |
| Hyaluronic acid |
|
| [69] |
| Agar |
|
| [86] |
| Pectin |
|
| [87] |
| Gellan gum |
|
| [78] |
| Polysaccharide | Derivatives | Modification in Properties | References |
|---|---|---|---|
| Chitosan | Carboxymethyl chitosan | Enhanced solubility, water retention capacity and antioxidant activity | [45] |
| N-Trimethyl Chitosan | Increased mucoadhesive property | ||
| Thiolated Chitosan | High permeation, mucoadhesion, higher solubility at physiological pH, in situ gelling property | ||
| Polyethylene glycol (PEG)-grafted chitosan | Increased solubility over a wide range of pH, enhanced mucoadhesion | ||
| Hyaluronic acid (HA) | HA esters | Decreased water solubility of HA, with the aim to reduce its susceptibility to hyaluronidase degradation and enhance its in-situ residence time | [72,88] |
| HA amides | High grafting yield | ||
| Can be conjugated with various biocompatible polymers like PEG, Chitosan, Poly(lactide-co-glycolide) | Delivery carrier for various hydrophobic and hydrophilic drugs | ||
| Dextran | Dextran esters | Greater flocculation performance in acidic condition, enhanced melting behavior | [62] |
| Dialdehyde Dextran | Enhanced crosslinking activity which can strengthen the nanostructure of biopolymer-based nanocarriers | ||
| Gellan | Methacrylated derivatives | Enhanced mucoadhesive property | [89] |
| Starch | Hydroxyalkyl starches | Enhancement in solubility, ease of hydration and swelling power | [23,24] |
| Acetylated Starch | Imparts hydrophobicity, enhancement of thermoplastic character, retardation of crystallization and lowering of pasting temperature | ||
| Starch cross-linked with sodium trimetaphosphate | Imparts tolerance against extreme pH and high shear conditions | ||
| Cellulose | Citric acid cross-linked bacterial cellulose | Improvement in water absorption capacity(About 1.5 times higher) | [90,91,92] |
| Carboxymethyl cellulose acetate butyrate | Enhances hydrophobicity and thermoplastic behavior making it suitable for processing as scaffolds | ||
| 1,3 cycloadditions of porphyrin on cellulose | Induction of bactericidal activity | ||
| Pullulan | Grafting of methyl acrylate onto pullulan by copolymerization | Increase in hydrophobicity for drug delivery applications | [51,52] |
| Carboxymethylation of pullulan with sodium chloroacetate | Introduction of negative charge that prolongs the residence time of the polymeric matrix inside the body | ||
| Carrageenan | Methacrylated carrageenan | Confers the ability to be photo crosslinked that allows easy tailorability of viscosity, swelling ratio, elastic moduli and pore size distribution | [55] |
| Pectin | Methoxylated derivatives | Altered solubility, gel forming ability, conditions required for gelation, gelling temperature, and gel properties | [93] |
| Acetylated derivatives | Emulsifying and stabilizing property | ||
| Amidated pectin | Good gelling property and reduced sensitivity against cations and pH |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salave, S.; Rana, D.; Sharma, A.; Bharathi, K.; Gupta, R.; Khode, S.; Benival, D.; Kommineni, N. Polysaccharide Based Implantable Drug Delivery: Development Strategies, Regulatory Requirements, and Future Perspectives. Polysaccharides 2022, 3, 625-654. https://doi.org/10.3390/polysaccharides3030037
Salave S, Rana D, Sharma A, Bharathi K, Gupta R, Khode S, Benival D, Kommineni N. Polysaccharide Based Implantable Drug Delivery: Development Strategies, Regulatory Requirements, and Future Perspectives. Polysaccharides. 2022; 3(3):625-654. https://doi.org/10.3390/polysaccharides3030037
Chicago/Turabian StyleSalave, Sagar, Dhwani Rana, Amit Sharma, K. Bharathi, Raghav Gupta, Shubhangi Khode, Derajram Benival, and Nagavendra Kommineni. 2022. "Polysaccharide Based Implantable Drug Delivery: Development Strategies, Regulatory Requirements, and Future Perspectives" Polysaccharides 3, no. 3: 625-654. https://doi.org/10.3390/polysaccharides3030037