Production of the Polysaccharide Pullulan by Aureobasidium pullulans Cell Immobilization
Abstract
:1. Introduction
2. Types of Cell Immobilization
3. Pullulan Production by Carrier-Binding Cell Immobilization
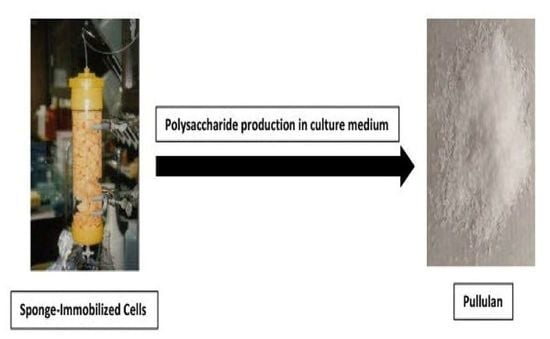
3.1. Polyurethane Foam, Sponge and Diatomaceous Earth as Solid Supports
3.2. Ion Exchange Resins as Solid Supports
3.3. Zeolite and Plastic Composites as Solid Supports
4. Pullulan Production Using Cell Immobilization Using Entrapment
4.1. Polyurethane Foam and Polyvinyl Alcohol as Agents to Entrap Fungal Cells for Pullulan Production
4.2. Calcium Alginate and Agar as Cell Entrapment Agents for Pullulan Production
4.3. Agarose, Carrageenan and Chitosan as Cell Entrapment Agents for Pullulan Production
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zajic, J.E.; LeDuy, A. Flocculant and chemical properties of polysaccharide from Pullularia pullulans. Appl. Microbiol. 1973, 25, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Kaur, N.; Rana, V.; Kennedy, J.F. Pullulan: A novel molecule for biomedical applications. Carbohydr. Polym. 2017, 171, 102–121. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Jia, S.-L.; Liu, G.-L.; Chi, Z.; Chi, Z.-M. Aureobasidium spp. and their applications in biotechnology. Process. Biochem. 2022, 116, 72–83. [Google Scholar] [CrossRef]
- Ueda, S.; Fujita, K.; Komatsu, K.; Nakashima, Z. Polysaccharide produced by the genus Pullularia. I. Production of polysaccharide by growing cells. Appl. Microbiol. 1963, 11, 211–215. [Google Scholar] [CrossRef]
- Leal-Serrano, G.; Ruperez, P.; Leal, J.A. Acidic polysaccharide from Aureobasidium pullulans. Trans. Brit. Mycol. Soc. 1980, 75, 57–62. [Google Scholar] [CrossRef]
- Bouveng, H.O.; Kiessling, H.; Lindberg, B.; McKay, J. Polysaccharides elaborated by Pullularia pullulans. I. The neutral glucan synthesized from sucrose solutions. Acta Chem. Scand. 1962, 16, 615–622. [Google Scholar] [CrossRef] [Green Version]
- Sowa, W.; Blackwood, A.C.; Adams, G.A. Neutral extracellular glucan of Pullularia pullulans (de Bary) Berkhout. Can. J. Chem. 1963, 41, 2314–2319. [Google Scholar] [CrossRef]
- Catley, B.J. Pullulan, a relationship between molecular weight and fine structure. FEBS Lett. 1970, 10, 190–193. [Google Scholar] [CrossRef] [Green Version]
- Sugumaran, K.R.; Ponnusami, S. Pullulan: A novel molecule for biomedical applications. Carbohydr. Polym. 2017, 173, 573–591. [Google Scholar]
- Oguzhan, P.; Yangilar, F. Pullulan: Production and usage in food industry. Afr. J. Food Sci. Technol. 2013, 4, 57–63. [Google Scholar]
- Prasongsuk, S.; Lotrakul, P.; Ali, I.; Bankeeree, W.; Punnapayak, H. The current status of Aureobasidium pullulans in biotechnology. Folia Microbiol. 2018, 63, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Kaur, N.; Rana, V.; Kennedy, J.F. Pullulan production from agro-industrial waste and its applications in food industry: A review. Carbohydr. Polym. 2019, 217, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.B.; Mir, S.A.; Khanday, F.A.; Masodi, F.A. Advances in pullulan production from agro-based wastes by Aureobasidium pullulans and its applications. Innov. Food Sci. Emerg. Technol. 2021, 74, 102846. [Google Scholar] [CrossRef]
- Jiang, L. Optimization of fermentation conditions for pullulan production using response surface methodology. Carbohydr. Polym. 2010, 79, 414–417. [Google Scholar] [CrossRef]
- Catley, B.J. Utilization of carbon sources by Pullularia pullulans for the elaboration of extracellular polysaccharides. Appl. Microbiol. 1971, 22, 641–649. [Google Scholar] [CrossRef]
- Leathers, T.D.; Nofsinger, G.W.; Kurtzman, C.P.; Bothast, R.J. Pullulan production by color variant strains of Aureobasidium pullulans. J. Ind. Microbiol. Biotechnol. 1988, 3, 231–239. [Google Scholar]
- West, T.P.; Reed-Hamer, B. Ability of Aureobasidium pullulans to synthesize pullulan upon selected sources of carbon and nitrogen. Microbios 1991, 67, 117–124. [Google Scholar]
- West, T.P.; Reed-Hamer, B. Polysaccharide production by a reduced pigmentation mutant of the fungus Aureobasidium pullulans. FEMS Microbiol. Lett. 1993, 113, 345–349. [Google Scholar] [CrossRef]
- West, T.P.; Reed-Hamer, B. Elevated polysaccharide production by mutants of the fungus Aureobasidium pullulans. FEMS Microbiol. Lett. 1994, 124, 167–172. [Google Scholar] [CrossRef]
- Catley, B.J. Role of pH and nitrogen limitation in the elaboration of the extracellular polysaccharide pullulan by Pullularia pullulans. Appl. Microbiol. 1971, 22, 650–654. [Google Scholar] [CrossRef]
- West, T.P.; Reed-Hamer, B. Effect of pH on pullulan production relative to carbon source and yeast extract composition of growth medium. Microbios 1993, 75, 75–82. [Google Scholar]
- Catley, B.J. The rate of elaboration of the extracellular polysaccharide, pullulan, during growth of Pullularia pullulans. J. Gen. Microbiol. 1973, 78, 33–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed-Hamer, B.; West, T.P. Effect of complex nitrogen sources on pullulan production relative to carbon source. Microbios 1994, 80, 83–90. [Google Scholar]
- West, T.P.; Reed-Hamer, B. Effect of oils and surfactants on pullulan production relative to nitrogen source. Microbios 1995, 83, 249–259. [Google Scholar]
- West, T.P.; Reed-Hamer, B. Pullulan production by Aureobasidium pullulans grown on ethanol stillage as a nitrogen source. Microbios 1996, 88, 7–18. [Google Scholar]
- West, T.P.; Reed-Hamer, B. Pullulan production by Aureobasidium pullulans grown on corn steep solids as a nitrogen source. Microbios 1997, 92, 171–181. [Google Scholar]
- West, T.P.; Reed-Hamer, B. Effect of nitrogen source on pullulan production by Aureobasidium pullulans grown in a batch bioreactor. Microbios 1999, 99, 147–159. [Google Scholar]
- Wang, D.; Chen, F.; Wei, G.; Jiang, M.; Dong, M. The mechanism of improved pullulan production by nitrogen limitation in batch culture of Aureobasidium pullulans. Carbohydr. Polym. 2015, 127, 325–331. [Google Scholar] [CrossRef] [PubMed]
- West, T.P.; Reed-Hamer, B. Effect of temperature on pullulan production in relation to carbon source. Microbios 1993, 75, 261–268. [Google Scholar]
- He, C.; Zhang, Z.; Zhang, Y.; Wang, G.; Wang, C.; Wang, D.; Wei, G. Efficient pullulan production by Aureobasidium pullulans using cost-effective substrates. Int. J. Biol. Macromol. 2021, 186, 544–553. [Google Scholar] [CrossRef]
- Reeslev, M.; Nielsen, J.C.; Olsen, J.; Jensen, B.; Jacobsen, T. Effect of pH and the initial concentration of yeast extract on regulation of dimorphism and exopolysaccharide formation of Aureobasidium pullulans in batch culture. Mycol. Res. 1991, 95, 220–226. [Google Scholar] [CrossRef]
- West, T.P.; Reed-Hamer, B. Influence of vitamins and mineral salts upon pullulan synthesis by Aureobasidium pullulans. Microbios 1992, 71, 115–123. [Google Scholar]
- West, T.P.; Strohfus, B. Effect of manganese on polysaccharide production and cellular pigmentation in the fungus Aureobasidium pullulans. World J. Microbiol. Biotechnol. 1997, 13, 233–235. [Google Scholar] [CrossRef]
- West, T.P. Fungal production of the polysaccharide pullulan from a plant hydrolysate. Z. Naturforsch. C 2017, 72, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.E., II; West, T.P. Effect of yeast extract addition to a mineral salts medium containing hydrolyzed plant xylan on fungal pullulan production. Z. Naturforsch. C 2018, 73, 319–323. [Google Scholar] [CrossRef]
- Hilares, R.T.; Resende, J.; Orsi, C.A.; Ahmed, M.A.; Lacerda, T.M.; DaSilva, S.S.; Santos, J.C. Exopolysaccharide (pullulan) production from sugarcane bagasse hydrolysate aiming to favor the development of biorefineries. Int. J. Biol. Macromol. 2019, 127, 169–177. [Google Scholar] [CrossRef]
- Rho, D.; Mulchandsani, A.; Luong, J.H.T.; LeDuy, A. Oxygen requirement in pullulan fermentation. Appl. Microbiol. Biotechnol. 1988, 28, 361–366. [Google Scholar] [CrossRef]
- Wecker, A.; Onken, U. Influence of dissolved oxygen concentration and shear rate on the production of pullulan by Aureobasidium pullulans. Biotechnol. Lett. 1991, 13, 155–160. [Google Scholar] [CrossRef]
- Perkins, C.; Siddiqui, S.; Puri, M.; Demain, A.L. Biotechnological applications of microbial bioconversions. Crit. Rev. Biotechnol. 2016, 36, 1050–1065. [Google Scholar] [CrossRef]
- Zur, J.; Wojcieszyska, D.; Guzik, U. Metabolic responses of bacterial cells to immobilization. Molecules 2016, 21, 958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno-García, J.; García-Martínez, T.; Mauricio, J.C.; Moreno, J. Yeast immobilization systems for alcoholic wine fermentations: Actual trends and future perspectives. Front. Microbiol. 2018, 9, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, N.; Renugopalakrishnan, V.; Liepmann, D.; Paulmurugand, R.; Malhotra, B.D. Cell-based biosensors: Recent trends, challenges and future perspectives. Biosens. Bioelectron. 2019, 141, 111435. [Google Scholar] [CrossRef] [PubMed]
- Vitolo, M. Overview on immobilization. World J. Pharm. Res. 2021, 10, 2121–2145. [Google Scholar]
- Lapponi, M.J.; Méndez, M.B.; Trelles, J.A.; Rivero, C.W. Cell immobilization strategies for biotransformations. Curr. Opin. Green Sustain. 2022, 33, 100565. [Google Scholar] [CrossRef]
- Gainer, J.L.; Kirwan, D.J. Immobilization of microorganisms. Ann. N. Y. Acad. Sci. 1984, 434, 465–467. [Google Scholar] [CrossRef]
- Cheetham, P.S.; Garrett, J.C.; Clark, J. Isomaltulose production using immobilized cells. Biotechnol. Bioeng. 1985, 27, 471–481. [Google Scholar] [CrossRef]
- Bar, R.; Gainer, J.L.; Kirwan, D.J. Ethanol fermentation by ionically adsorbed Zymomomas mobilis: Environmental effects on cell immobilization. Biotechnol. Bioeng. 1987, 29, 1045–1049. [Google Scholar] [CrossRef]
- Monsan, P.; Durand, G.; Navarro, J.M. Immobilization of microbial cells by adsorption to solid supports. Methods Enzymol. 1987, 135, 307–318. [Google Scholar]
- Mozes, N.; Marchal, F.; Hermesse, M.P.; Van Haecht, J.L.; Reuliaux, L.; Leonard, A.J.; Rouxhet, P.G. Immobilization of microorganisms by adhesion: Interplay of electrostatic and nonelectrostatic interaction. Biotechnol. Bioeng. 1987, 30, 439–450. [Google Scholar] [CrossRef]
- Dunn, I.J. Methods for selecting and growing mixed cultures in biofilm fluidized sand beds. Methods Enzymol. 1987, 135, 300–307. [Google Scholar]
- Tisnadjaja, D.; Gutierrez, N.A.; Maddox, I.S. Citric acid production in a bubble-column reactor using cells of the yeast Candida guilliermondii immobilized by adsorption onto sawdust. Enzyme Microb. Technol. 1996, 19, 343–347. [Google Scholar] [CrossRef]
- Iqbal, M.; Saeed, A. Novel method for cell immobilization and its application for production of organic acid. Lett. Appl. Microbiol. 2005, 40, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.K.; Banerjee, P.C. Submerged production of oxalic acid from glucose by immobilized Aspergillus niger. Process. Biochem. 2005, 40, 1605–1610. [Google Scholar] [CrossRef]
- Romaskevic, T.; Budriene, S.; Pielichowski, K.; Pielichowski, J. Application of polyurethane-based materials for immobilization of enzymes and cells: A review. Chemija 2006, 17, 74–89. [Google Scholar]
- Saeed, A.; Iqbal, M. Loofa (Luffa cylindrica) sponge: Review of development of the biomatrix as a tool for biotechnological applications. Biotechnol. Prog. 2013, 29, 573–600. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Mozafari, M. Biocompatibility of alumina-based biomaterials—A review. J. Cell Physiol. 2019, 234, 3321–3335. [Google Scholar] [CrossRef] [PubMed]
- Terpou, A.; Ganatsios, V.; Kanellaki, M.; Koutinas, A.A. Entrapped psychrotolerant yeast cells within pin dust for low temperature wine making: Impact on wine quality. Microorganisms 2020, 8, 764. [Google Scholar] [CrossRef]
- Luong, J.H.T. Cell immobilization in ĸ-carrageenan for ethanol production. Biotechnol. Bioeng. 1985, 27, 1652–1661. [Google Scholar] [CrossRef] [PubMed]
- Doran, P.M.; Bailey, J.E. Effects of immobilization on growth, fermentation properties and macromolecular composition of Saccharomyces cerevisiae attached to gelatin. Biotechnol. Bioeng. 1986, 28, 73–87. [Google Scholar] [CrossRef]
- Dainty, A.L.; Goulding, K.H.; Robinson, P.K.; Simpkins, I.; Trevan, M.D. Stability of alginate-immobilized algal cells. Biotechnol. Bioeng. 1986, 28, 210–216. [Google Scholar] [CrossRef]
- Nilsson, K.; Brodelius, P.; Mosbach, K. Entrapment of microbial and plant cells in beaded polymers. Methods Enzymol. 1987, 135, 222–230. [Google Scholar] [PubMed]
- Vorlop, K.D.; Klein, J. Entrapment of microbial cells in chitosan. Methods Enzymol. 1987, 135, 259–268. [Google Scholar] [PubMed]
- Guo, X.; Wang, Y.; Qin, Y.; Shen, P.; Peng, Q. Structures, properties and applications of alginic acid: A review. Int. J. Biol. Macromol. 2020, 162, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Ercole, A.; Raganati, F.; Salatino, P.; Marzocchella, A. Continuous succinic acid production by immobilized cells of Actinobacillus succinogenes in a fluidized bed reactor: Entrapment in alginate beads. Biochem. Eng. J. 2021, 169, 107968. [Google Scholar] [CrossRef]
- Soares, R.C.; Zangirolami, T.C.; Giordano, R.L.C.; Demeke, M.M.; Thevelein, J.M.; Milessi, T.S. Cell immobilization using alginate-based beads as a protective technique against stressful conditions of hydrolysates for 2G ethanol production. Polymers 2022, 14, 2400. [Google Scholar] [CrossRef]
- Mulchandani, A.; Luong, J.H.T.; LeDuy, A. Biosynthesis of pullulan using immobilized Aureobasidium pullulans cells. Biotechnol. Bioeng. 1989, 33, 306–312. [Google Scholar] [CrossRef]
- Singh, R.; Gaur, R.; Jamal, F.; Gaur, M.K. A novel fermentor system for continuous production of pullulan. Afr. J. Biotechnol. 2011, 10, 9839–9846. [Google Scholar]
- West, T.P.; Strohfus, B.R.-H. Polysaccharide production by sponge-immobilized cells of the fungus Aureobasidium pullulans. Lett. Appl. Microbiol. 1996, 22, 162–164. [Google Scholar] [CrossRef]
- West, T.P.; Reed-Hamer, B. Use of adsorption in immobilizing fungal cells for pullulan production. Microbios 1996, 85, 117–125. [Google Scholar]
- West, T.P.; Strohfus, B. Fungal cell immobilization on ion exchange resins for pullulan production. Microbios 1996, 88, 177–187. [Google Scholar]
- West, T.P. Pullulan production by Aureobasidium pullulans ATCC 201253 cells adsorbed onto cellulose anion and cation exchangers. ISRN Microbiol. 2012, 2012, 140951. [Google Scholar] [CrossRef] [Green Version]
- West, T.P.; Reed, B. Effect of adsorption pH on column bioreactor production of the polysaccharide pullulan using cells immobilized on an ion-exchange resin. Curr. Top. Biotechnol. 2004, 1, 89–94. [Google Scholar]
- West, T.P. Pullulan production by Aureobasidium pullulans cells immobilized on ECTEOLA-cellulose. Ann. Microbiol. 2010, 60, 763–766. [Google Scholar] [CrossRef]
- West, T.P.; Strohfus, B. Fungal cell immobilization on zeolite for pullulan production. Microbios 1997, 91, 121–130. [Google Scholar]
- Cheng, K.-C.; Demirci, A.; Catchmark, J.M. Effects of plastic composite support and pH profiles on pullulan production in a biofilm reactor. Appl. Microbiol. Biotechnol. 2010, 86, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.-C.; Demirci, A.; Catchmark, J.M. Advances in biofilm reactors for production of value-added products. Appl. Microbiol. Biotechnol. 2010, 87, 445–456. [Google Scholar] [CrossRef]
- Cheng, K.-C.; Demirci, A.; Catchmark, J.M. Continuous fermentation in a biofilm reactor. Appl. Microbiol. Biotechnol. 2011, 90, 921–927. [Google Scholar] [CrossRef]
- Senko, O.V.; Efremenko, E.N. Highly concentrated populations of Aureobasidium pullulans cells in biocatalytic pullulan production processes. Catal. Ind. 2017, 9, 344–348. [Google Scholar] [CrossRef]
- Urkut, Z.; Dagbagli, S.; Goksungur, Y. Optimization of pullulan production using Ca-alginate-immobilized Aureobasidium pullulans by response surface methodology. J. Chem. Technol. Biotechnol. 2007, 82, 837–846. [Google Scholar] [CrossRef]
- West, T.P. Effect of carbon source on polysaccharide production by alginate-entrapped Aureobasidium pullulans ATCC 42023 cells. J. Basic Microbiol. 2011, 51, 673–677. [Google Scholar] [CrossRef]
- West, T.P.; Strohfus, B. Polysaccharide production by Aureobasidium pullulans cells immobilized by entrapment. Microbiol. Res. 1998, 153, 253–256. [Google Scholar] [CrossRef]
- West, T.P.; Strohfus, B. Polysaccharide production by immobilized Aureobasidium pullulans cells in batch bioreactors. Microbiol. Res. 2001, 156, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Lebrun, L.; Junter, G.-A.; Jouenne, T.; Mignot, L. Exopolysaccharide production by free and immobilized microbial cultures. Enzyme Microb. Technol. 1994, 16, 1048–1054. [Google Scholar] [CrossRef]
- West, T.P. Exopolysaccharide production by entrapped cells of the fungus Aureobasidium pullulans ATCC 201253. J. Basic Microbiol. 2000, 40, 397–401. [Google Scholar] [CrossRef]
- West, T.P. Pullulan production by Aureobasidium pullulans cells immobilized in chitosan beads: Fungal pullulan production by immobilized cells. Folia Microbiol. 2011, 56, 335–338. [Google Scholar] [CrossRef] [PubMed]
 ) connected through α-D-(1→6) bonds on its terminal glucose residues.
) connected through α-D-(1→6) bonds on its terminal glucose residues.
 ) connected through α-D-(1→6) bonds on its terminal glucose residues.
) connected through α-D-(1→6) bonds on its terminal glucose residues.
| Carrier Support | Strain | Carbon Source | Adsorption pH/Growth Conditions | Cycles of Use | Pullulan (g/L) First Use | Pullulan (g/L) Final Use | Reference |
|---|---|---|---|---|---|---|---|
| Polyurethane foam | Wild-type | Sucrose | 5.5/48 h, 42 °C | 18 | 37.0 | 37.0 | [67] |
| Sponge | ATCC 42023 | Maltose corn syrup | 6.0/168 h, 30 °C | 3 | 4.5 | 5.6 | [68] |
| Sponge | ATCC 42023 | Sucrose | 6.0/168 h, 30 °C | 3 | 5.4 | 6.2 | [68] |
| Sponge | ATCC 42023 | Glucose | 6.0/168 h, 30 °C | 3 | 5.4 | 6.7 | [68] |
| Diatomaceous earth | ATCC 42023 | Maltose corn syrup | 2.0/168 h, 30 °C | 2 | 7.0 | 5.3 | [69] |
| DEAE-cellulose | ATCC 42023 | Maltose corn syrup | 2.0/168 h, 30 °C | 2 | 4.5 | 5.8 | [69] |
| DEAE-cellulose | ATCC 201253 | Maltose corn syrup | 2.0/168 h, 30 °C | 2 | 5.6 | 3.9 | [70] |
| TEAE-cellulose | ATCC 201253 | Maltose corn syrup | 2.0/168 h, 30 °C | 2 | 6.4 | 6.9 | [70] |
| TEAE-cellulose | ATCC 201253 | Maltose corn syrup | 4.0/168 h, 30 °C | 2 | 5.7 | 5.1 | [71] |
| TEAE-cellulose | ATCC 201253 | Maltose corn syrup | 2.0/336 h, 25 °C | 2 | 1.7 | 2.6 | [72] |
| TEAE-cellulose | ATCC 201253 | Maltose corn syrup | 6.0/336 h, 25 °C | 2 | 3.6 | 4.4 | [72] |
| TEAE-cellulose | ATCC 201253 | Maltose corn syrup | 7.5/336 h, 25 °C | 2 | 6.4 | 0.6 | [72] |
| Phosphocellulose | ATCC 201253 | Maltose corn syrup | 2.0/168 h, 30 °C | 2 | 4.6 | 4.0 | [70] |
| Phosphocellulose | ATCC 201253 | Maltose corn syrup | 4.0/168 h, 30 °C | 2 | 3.6 | 4.3 | [71] |
| ECTEOLA-cellulose | ATCC 42023 | Maltose corn syrup | 2.0/168 h, 30 °C | 2 | 5.5 | 5.0 | [73] |
| Zeolite | ATCC 201253 | Maltose corn syrup | 4.0/168 h, 30 °C | 2 | 6.2 | 7.2 | [74] |
| Zeolite | ATCC 201253 | Maltose corn syrup | 5.0/168 h, 30 °C | 2 | 6.3 | 6.9 | [74] |
| Plastic composite | ATCC 201253 | Glucose | 2.0/168 h, 30 °C | 1 | 0.0 | 32.9 | [75] |
| Plastic composite | ATCC 201253 | Glucose | 5.0/168 h, 30 °C | 1 | 0.0 | 25.2 | [75] |
| Entrapment Agent | Strain | Carbon Source | Growth Conditions | Cycles of Use | Pullulan (g/L) First Use | Pullulan (g/L) Final Use | Reference |
|---|---|---|---|---|---|---|---|
| Polyurethane foam | ATCC 42023 | Sucrose | 96 h, 28 °C | 4 | 12.0 | 18.0 | [66] |
| Polyvinyl alcohol | Y-4137 | Glucose | 45 h, 26 °C | 16 | 10.0 | 9.0 | [78] |
| 2% Calcium alginate | P56 | Sucrose | 120 h, 28 °C | 7 | 21.0 | 10.0 | [79] |
| 5% Calcium alginate | ATCC 42023 | Glucose | 168 h, 30 °C | 2 | 7.6 | 4.0 | [80] |
| 5% Calcium alginate | ATCC 42023 | Sucrose | 168 h, 30 °C | 2 | 10.3 | 4.0 | [80] |
| 5% Calcium alginate | ATCC 201253 | Maltose corn syrup | 168 h, 30 °C | 2 | 4.3 | 4.8 | [81] |
| 4% Agar | ATCC 201253 | Maltose corn syrup | 168 h, 30 °C | 2 | 4.4 | 6.8 | [81] |
| 5% Calcium alginate | ATCC 201253 | Maltose corn syrup | 168 h, 30 °C | 2 | 3.4 | 5.6 | [82] |
| 4% Agar | ATCC 201253 | Maltose corn syrup | 168 h, 30 °C | 2 | 3.9 | 4.0 | [82] |
| 4% Agarose | ATCC 201253 | Maltose corn syrup | 168 h, 30 °C | 2 | 4.4 | 5.6 | [84] |
| 3% Carrageenan | ATCC 201253 | Maltose corn syrup | 168 h, 30 °C | 2 | 4.7 | 4.8 | [84] |
| 1% Chitosan | ATCC 201253 | Maltose corn syrup | 168 h, 30 °C | 2 | 6.7 | 5.4 | [85] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
West, T.P. Production of the Polysaccharide Pullulan by Aureobasidium pullulans Cell Immobilization. Polysaccharides 2022, 3, 544-555. https://doi.org/10.3390/polysaccharides3030032
West TP. Production of the Polysaccharide Pullulan by Aureobasidium pullulans Cell Immobilization. Polysaccharides. 2022; 3(3):544-555. https://doi.org/10.3390/polysaccharides3030032
Chicago/Turabian StyleWest, Thomas P. 2022. "Production of the Polysaccharide Pullulan by Aureobasidium pullulans Cell Immobilization" Polysaccharides 3, no. 3: 544-555. https://doi.org/10.3390/polysaccharides3030032