Agave By-Products: An Overview of Their Nutraceutical Value, Current Applications, and Processing Methods
Abstract
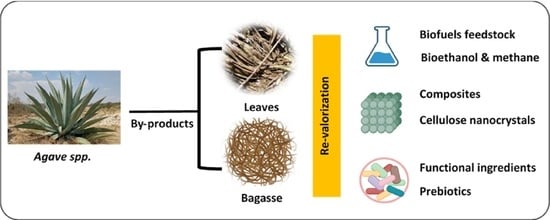
:1. Introduction
2. Agave By-Products
3. Chemical Composition of the Main Agave By-Products
4. Main Applications of Agave By-Products
4.1. Functional Potential
4.1.1. Antioxidant and Anti-Inflammatory Activity
4.1.2. Antibacterial and Antifungal Activity
4.1.3. By-Products as Food Ingredients
4.2. Agave as a Potential Biofuel Feedstock
4.2.1. Bioethanol
4.2.2. Hydrogen and Methane Production
4.3. Nanocomposites and Nanocrystals
5. Processes and Technologies for the Treatment of Agave By-Products
5.1. Chemical Pretreatments
5.2. Enzymatic Saccharification and Fermentation
5.3. Ultrasound
5.4. Microwaves
5.5. Other Processes
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bremer, B.; Bremer, K.; Chase, M.W.; Fay, M.F.; Reveal, J.L.; Bailey, L.H.; Angiosperm Phylogeny Group. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot. J. Linn. Soc. 2009, 161, 105–121. [Google Scholar] [CrossRef] [Green Version]
- García Mendoza, A. Distribution of Agave (Agavaceae) in Mexico. Cactus. Succul. J. 2002, 74, 177–187. [Google Scholar]
- García Mendoza, A.J.; Franco Martínez, I.S.; Sandoval Gutiérrez, D. Cuatro especies nuevas de Agave (Asparagaceae, Agavoideae) del sur de México. Acta Bot. Mex. 2019. [Google Scholar] [CrossRef]
- de Lourdes Pérez-Zavala, M.; Hernández-Arzaba, J.C.; Bideshi, D.K.; Barboza-Corona, J.E. Agave: A natural renewable resource with multiple applications. J. Sci. Food Agric. 2020, 100, 5324–5333. [Google Scholar] [CrossRef]
- López-Romero, J.C.; Ayala-Zavala, J.F.; González-Aguilar, G.A.; Peña-Ramos, E.A.; González-Ríos, H. Biological activities of Agave by-products and their possible applications in food and pharmaceuticals. J. Sci. Food Agric. 2018, 98, 2461–2474. [Google Scholar] [CrossRef] [PubMed]
- Iñiguez-Covarrubias, G.; Díaz-Teres, R.; Sanjuan-Duenas, R.; Anzaldo-Hernández, J.; Rowell, R.M. Utilization of by-products from the tequila industry. Part 2: Potential value of Agave tequilana Weber azul leaves. Bioresour. Technol. 2001, 77, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Samilpa, A.; Reyes-Agüero, J.A.; Álvarez-Fuentes, G.; Aguirre Rivera, J.R.; Van’t Hooft, A. Physical Characterization of the Fibers of Agave salmiana and A. mapisaga (Asparagaceae) from the Mezquital Valley, Mexico. J. Nat. Fibers 2020, 11, 1–8. [Google Scholar] [CrossRef]
- Thamae, T.; Baillie, C. Influence of fibre extraction method, alkali and silane treatment on the interface of Agave americana waste HDPE composites as possible roof ceilings in Lesotho. Compos. Interfaces 2007, 14, 821–836. [Google Scholar] [CrossRef]
- Langhorst, A.; Paxton, W.; Bollin, S.; Frantz, D.; Burkholder, J.; Kiziltas, A. Heat-treated blue agave fiber composites. Compos. Part B Eng. 2019, 165, 712–724. [Google Scholar] [CrossRef]
- Langhorst, A.E.; Burkholder, J.; Long, J.; Thomas, R.; Kiziltas, A.; Mielewski, D. Blue-agave fiber-reinforced polypropylene composites for automotive applications. BioResources 2018, 138, 20–35. [Google Scholar] [CrossRef]
- Prasad, L.; Singh, V.; Patel, R.V.; Yadav, A.; Kumar, V.; Winczek, J. Physical and Mechanical Properties of Rambans (Agave) Fiber Reinforced with Polyester Composite Materials. J. Nat. Fibers 2021. [Google Scholar] [CrossRef]
- Huerta-Cardoso, O.; Durazo-Cardenas, I.; Longhurst, P.; Simms, N.J.; Encinas-Oropesa, A. Fabrication of Agave Tequilana Bagasse/PLA Composite and Preliminary Mechanical Properties Assessment. Ind. Crop. Prod. 2020, 152, 112–523. [Google Scholar] [CrossRef]
- Cisneros-López, E.O.; Pérez-Fonseca, A.A.; González-García, Y.; Ramírez-Arreola, D.E.; González-Núñez, R.; Rodrigue, D.; Robledo-Ortíz, J.R. Polylactic Acid–Agave Fiber Biocomposites Produced by Rotational Molding: A Comparative Study with Compression Molding. Adv. Polym. Technol. 2018, 37, 2528–2540. [Google Scholar] [CrossRef]
- Torres-Tello, E.V.; Robledo-Ortíz, J.R.; González-García, Y.; Pérez-Fonseca, A.A.; Jasso-Gastinel, C.F.; Mendizábal, E. Effect of Agave Fiber Content in the Thermal and Mechanical Properties of Green Composites Based on Polyhydroxybutyrate or Poly (Hydroxybutyrate-Co-Hydroxyvalerate). Ind. Crop. Prod. 2017, 99, 117–125. [Google Scholar] [CrossRef]
- Smith, M.K.M.; Paleri, D.M.; Abdelwahab, M.; Mielewski, D.F.; Misra, M.; Mohanty, A.K. Sustainable Composites from Poly(3-Hydroxybutyrate) (PHB) Bioplastic and Agave Natural Fibre. Green Chem. 2020, 22, 3906–3916. [Google Scholar] [CrossRef]
- Pérez-Fonseca, A.A.; Robledo-Ortíz, J.R.; Ramirez-Arreola, D.E.; Ortega-Gudiño, P.; Rodrigue, D.; González-Núñez, R. Effect of Hybridization on the Physical and Mechanical Properties of High Density Polyethylene-(Pine/Agave) Composites. Mater. Des. 2014, 64, 35–43. [Google Scholar] [CrossRef]
- Láinez, M.; Ruiz, H.A.; Arellano-Plaza, M.; Martínez-Hernández, S. Bioethanol Production from Enzymatic Hydrolysates of Agave salmiana Leaves Comparing, S. cerevisiae and K. marxianus. Renew. Energy 2019, 138, 1127–1133. [Google Scholar] [CrossRef]
- Rijal, D.; Vancov, T.; McIntosh, S.; Ashwath, N.; Stanley, G.A. Process Options for Conversion of Agave Tequilana Leaves into Bioethanol. Ind. Crop. Prod. 2016, 84, 263–272. [Google Scholar] [CrossRef]
- Hernández-Salas, J.M.; Villa-Ramírez, M.S.; Veloz-Rendón, J.S.; Rivera-Hernández, K.N.; González-César, R.A.; Plascencia-Espinosa, M.A.; Trejo-Estrada, S.R. Comparative Hydrolysis and Fermentation of Sugarcane and Agave Bagasse. Bioresour. Technol. 2009, 100, 1238–1245. [Google Scholar] [CrossRef]
- Moran-Salazar, R.G.; Marino-Marmolejo, E.N.; Rodriguez-Campos, J.; Davila-Vazquez, G.; Contreras-Ramos, S.M. Use of Agave Bagasse for Production of an Organic Fertilizer by Pretreatment with Bjerkandera adusta and Vermicomposting with Eisenia fetida. Environ. Technol. 2016, 37, 1220–1231. [Google Scholar] [CrossRef]
- Ramirez-Cortina, C.R.; Alonso-Gutierrez, M.S.; Rigal, L. Valorizacion de Residuos Agroindustriales Del Tequila Para Alimentacion de Ruminates. Rev. Chapingo Ser Ciencias For y del Ambient 2012, 18, 449–457. [Google Scholar] [CrossRef]
- Yang, H.; Chen, J.; Chen, Q.; Wang, K.; Sun, R.C. The Synergic Relationship Between Xylan Removal and Enhanced Cellulose Digestibility for Bioethanol Production: Reactive Area, Crystallinity, and Inhibitation. Bioenergy Res. 2015, 8, 1847–1855. [Google Scholar] [CrossRef]
- Arreola-Vargas, J.; Ojeda-Castillo, V.; Snell-Castro, R.; Corona-González, R.I.; Alatriste-Mondragón, F.; Méndez-Acosta, H.O. Methane Production from Acid Hydrolysates of Agave tequilana Bagasse: Evaluation of Hydrolysis Conditions and Methane Yield. Bioresour. Technol. 2015, 181, 191–199. [Google Scholar] [CrossRef]
- Arreola-Vargas, J.; Flores-Larios, A.; González-Álvarez, V.; Corona-González, R.I.; Méndez-Acosta, H.O. Single and Two-Stage Anaerobic Digestion for Hydrogen and Methane Production from Acid and Enzymatic Hydrolysates of Agave tequilana Bagasse. Int. J. Hydrogen Energy 2016, 41, 897–904. [Google Scholar] [CrossRef]
- Galindo-Hernández, K.L.; Tapia-Rodríguez, A.; Alatriste-Mondragón, F.; Celis, L.B.; Arreola-Vargas, J.; Razo-Flores, E. Enhancing Saccharification of Agave tequilana Bagasse by Oxidative Delignification and Enzymatic Synergism for the Production of Hydrogen and Methane. Int. J. Hydrogen Energy 2018, 43, 22116–22125. [Google Scholar] [CrossRef]
- Flores-Gómez, C.A.; Escamilla Silva, E.M.; Zhong, C.; Dale, B.E.; Da Costa Sousa, L.; Balan, V. Conversion of Lignocellulosic Agave Residues into Liquid Biofuels Using an AFEXTM-Based Biorefinery. Biotechnol. Biofuels 2018, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pimienta, J.A.; Vargas-Tah, A.; López-Ortega, K.M.; Medina-López, Y.N.; Mendoza-Pérez, J.A.; Avila, S.; Singh, S.; Simmons, B.A.; Loaces, I.; Martinez, A. Sequential Enzymatic Saccharification and Fermentation of Ionic Liquid and Organosolv Pretreated Agave Bagasse for Ethanol Production. Bioresour. Technol. 2017, 225, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Rios-González, L.J.; Morales-Martínez, T.K.; Rodríguez-Flores, M.F.; Rodríguez-De la Garza, J.A.; Castillo-Quiroz, D.; Castro-Montoya, A.J.; Martinez, A. Autohydrolysis Pretreatment Assessment in Ethanol Production from Agave Bagasse. Bioresour. Technol. 2017, 242, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, B.D.; Alviano, D.S.; Barreto, D.W.; Coelho, M.A.Z. Functional Properties of Saponins from Sisal (Agave sisalana) and Juá (Ziziphus joazeiro): Critical Micellar Concentration, Antioxidant and Antimicrobial Activities. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 436, 736–743. [Google Scholar] [CrossRef]
- Ribeiro, B.D.; Barreto, D.W.; Coelho, M.A.Z. Use of Micellar Extraction and Cloud Point Preconcentration for Valorization of Saponins from Sisal (Agave sisalana) Waste. Food Bioprod. Process. 2015, 94, 601–609. [Google Scholar] [CrossRef]
- Santos-Zea, L.; Gutiérrez-Uribe, J.A.; Benedito, J. Effect of Ultrasound Intensification on the Supercritical Fluid Extraction of Phytochemicals from Agave Salmiana Bagasse. J. Supercrit. Fluids 2019, 144, 98–107. [Google Scholar] [CrossRef]
- Carranza, C.O.; Fernandez, A.Á.; Bustillo Armendáriz, G.R.; López-Munguía, A. Processing of Fructans and Oligosaccharides from Agave Plants. In Processing and Impact on Active Components in Food; Preedy, V., Ed.; Academic Press: Piscataway, NJ, USA, 2015; pp. 121–129. [Google Scholar] [CrossRef]
- Ramos-Hernández, J.A.; Ragazzo-Sanchez, J.A.; Calderón-Santoyo, M.; Ortiz-Basurto, R.I.; Prieto, C.; Lagaron, J.M. Use of Electrosprayed Agave Fructans as Nanoencapsulating Hydrocolloids for Bioactives. Nanomaterials 2018, 8, 868. [Google Scholar] [CrossRef] [Green Version]
- Contreras-Hernández, M.G.; Ochoa-Martínez, L.A.; Rutiaga-Quiñones, J.G.; Rocha-Guzmán, N.E.; Lara-Ceniceros, T.E.; Contreras-Esquivel, J.C.; Prado Barragán, L.A.; Rutiaga-Quiñones, O.M. Effect of Ultrasound Pretreatment on the Physicochemical Composition of Agave durangensis Leaves and Potential Enzyme Production. Bioresour. Technol. 2018, 249, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Morán-Velázquez, D.C.; Monribot-Villanueva, J.L.; Bourdon, M.; Tang, J.Z.; López-Rosas, I.; Maceda-López, L.F.; Villalpando-Aguilar, J.L.; Rodríguez-López, L.; Gauthier, A.; Trejo, L.; et al. Unravelling Chemical Composition of Agave Spines: News from Agave Fourcroydes Lem. Plants 2020, 9, 1642. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Pan, X. Pretreatment of Agave Americana Stalk for Enzymatic Saccharification. Bioresour. Technol. 2012, 126, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Perez-Espana, V.H.; Cuervo-Parra, J.A.; Paz-Camacho, C.; Morales-Ovando, M.A.; Gomez-Aldapa, C.A.; Rodriguez-Jimenes, G.C.; Robles-Olvera, V.J.; Lopez-Perez, P.A.; Romero-Cortes, T. General Characterization of Cuticular Membrane Isolated from Agave salmiana. Int. J. Bio-Resour. Stress Manag. 2019, 10, 46–52. [Google Scholar] [CrossRef]
- Arredondo, C.H.; Aguilar-Lira, G.; Perez-Silva, I.; Rodriguez, J.A.; Islas, G.; Hernandez, P. Characterization and Application of Agave salmiana Cuticle as Bio-Membrane in Low-Temperature Electrolyzer and Fuel Cells. Appl. Sci. 2019, 9, 4461. [Google Scholar] [CrossRef] [Green Version]
- Espino, E.; Cakir, M.; Domenek, S.; Román-Gutiérrez, A.D.; Belgacem, N.; Bras, J. Isolation and Characterization of Cellulose Nanocrystals from Industrial By-Products of Agave tequilana and Barley. Ind. Crop. Prod. 2014, 62, 552–559. [Google Scholar] [CrossRef]
- Robles, E.; Fernández-Rodríguez, J.; Barbosa, A.M.; Gordobil, O.; Carreño, N.L.V.; Labidi, J. Production of Cellulose Nanoparticles from Blue Agave Waste Treated with Environmentally Friendly Processes. Carbohydr. Polym. 2018, 183, 294–302. [Google Scholar] [CrossRef]
- El Oudiani, A.; Msahli, S.; Sakli, F. In-Depth Study of Agave Fiber Structure Using Fourier Transform Infrared Spectroscopy. Carbohydr. Polym. 2017, 164, 242–248. [Google Scholar] [CrossRef]
- Perez-Pimienta, J.A.; Lopez-Ortega, M.G.; Varanasi, P.; Stavila, V.; Cheng, G.; Singh, S.; Simmons, B.A. Comparison of the Impact of Ionic Liquid Pretreatment on Recalcitrance of Agave Bagasse and Switchgrass. Bioresour. Technol. 2013, 127, 18–24. [Google Scholar] [CrossRef]
- Equihua-Sánchez, M.; Barahona-Pérez, L.F. Physical and Chemical Characterization of Agave tequilana Bagasse Pretreated with the Ionic Liquid 1-Ethyl-3-Methylimidazolium Acetate. Waste Biom. Valoriz. 2019, 10, 1285–1294. [Google Scholar] [CrossRef]
- Wang, J.; Chio, C.; Chen, X.; Su, E.; Cao, F.; Jin, Y.; Qin, W. Efficient Saccharification of Agave Biomass Using Aspergillus niger Produced Low-Cost Enzyme Cocktail with Hyperactive Pectinase Activity. Bioresour. Technol. 2019, 272, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Delgadillo Ruíz, L.; Bañuelos Valenzuela, R.; Esparza Ibarra, E.L.; Gutiérrez Bañuelos, H.; Cabral Arellano, F.J.; Muro Reyes, A. Evaluación Del Perfil de Nutrientes de Bagazo de Agave Como Alternativa de Alimento Para Rumiantes. Rev. Mex. Ciencias Agrícolas 2018, 11, 2099. [Google Scholar] [CrossRef] [Green Version]
- Jones, A.M.; Zhou, Y.; Held, M.A.; Davis, S.C. Tissue Composition of Agave americana L. Yields Greater Carbohydrates from Enzymatic Hydrolysis Than Advanced Bioenergy Crops. Front. Plant Sci. 2020, 11, 654. [Google Scholar] [CrossRef] [PubMed]
- Perez-Pimienta, J.A.; Flores-Gómez, C.A.; Ruiz, H.A.; Sathitsuksanoh, N.; Balan, V.; da Costa Sousa, L.; Dale, B.E.; Singh, S.; Simmons, B.A. Evaluation of Agave Bagasse Recalcitrance Using AFEXTM, Autohydrolysis, and Ionic Liquid Pretreatments. Bioresour. Technol. 2016, 211, 216–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Varela, J.D.; Chanona-Pérez, J.J.; Calderón Benavides, H.A.; Cervantes Sodi, F.; Vicente-Flores, M. Effect of Ball Milling on Cellulose Nanoparticles Structure Obtained from Garlic and Agave Waste. Carbohydr. Polym. 2021, 255, 117347. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Reyes, M.; Caballero-Caballero, M.; HernáNdez-Gómez, L.H.; Urriolagoitia-Calderón, G. Chemical and Morphological Characterization of Agave angustifolia Bagasse Fibers. Bot. Sci. 2015, 93, 807–817. [Google Scholar] [CrossRef] [Green Version]
- Kestur, G.S.; Flores-Sahagun, T.H.S.; Dos Santos, L.P.; Dos Santos, J.; Mazzaro, I.; Mikowski, A. Characterization of Blue Agave Bagasse Fibers of Mexico. Compos. Part A Appl. Sci. Manuf. 2013, 45, 153–161. [Google Scholar] [CrossRef]
- Deshmukh, A.P.; Simpson, A.J.; Hadad, C.M.; Hatcher, P.G. Insights into the Structure of Cutin and Cutan from Agave americana Leaf Cuticle Using HRMAS NMR Spectroscopy. Org. Geochem. 2005, 36, 1072–1085. [Google Scholar] [CrossRef]
- Escobedo-García, S.; Salas-Tovar, J.A.; Flores-Gallegos, A.C.; Contreras-Esquivel, J.C.; González-Montemayor, Á.M.; López, M.G.; Rodríguez-Herrera, R. Functionality of Agave Bagasse as Supplement for the Development of Prebiotics-Enriched Foods. Plant Foods Hum. Nutr. 2020, 75, 96–102. [Google Scholar] [CrossRef]
- Martinez-Gutierrez, F.; Ratering, S.; Juárez-Flores, B.; Godinez-Hernandez, C.; Geissler-Plaum, R.; Prell, F.; Zorn, H.; Czermak, P.; Schnell, S. Potential Use of Agave Salmiana as a Prebiotic That Stimulates the Growth of Probiotic Bacteria. LWT 2017, 84, 151–159. [Google Scholar] [CrossRef]
- Moreno-Vilet, L.; Garcia-Hernandez, M.H.; Delgado-Portales, R.E.; Corral-Fernandez, N.E.; Cortez-Espinosa, N.; Ruiz-Cabrera, M.A.; Portales-Perez, D.P. In Vitro Assessment of Agave Fructans (Agave salmiana) as Prebiotics and Immune System Activators. Int. J. Biol. Macromol. 2014, 63. [Google Scholar] [CrossRef]
- Urías-Silvas, J.E.; Cani, P.D.; Delmée, E.; Neyrinck, A.; López, M.G.; Delzenne, N.M. Physiological Effects of Dietary Fructans Extracted from Agave tequilana Gto. and Dasylirion spp. Br. J. Nutr. 2008, 99, 254–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamora-Gasga, V.M.; Bello-Pérez, L.A.; Ortíz-Basurto, R.I.; Tovar, J.; Sáyago-Ayerdi, S.G. Granola Bars Prepared with Agave tequilana Ingredients: Chemical Composition and Invitro Starch Hydrolysis. LWT Food Sci. Technol. 2014, 56, 309–314. [Google Scholar] [CrossRef]
- Bouaziz, M.A.; Mokni, A.; Masmoudi, M.; Bchir, B.; Attia, H.; Besbes, S. Gelling Qualities of Water Soluble Carbohydrate from Agave americana L. Leaf Extracts. Food Biosci. 2020, 35, 100543. [Google Scholar] [CrossRef]
- Gibson, G.R. Fibre and Effects on Probiotics (the Prebiotic Concept). Clin. Nutr. Suppl. 2004, 1, 25–31. [Google Scholar] [CrossRef]
- López-Romero, J.C.; Ayala-Zavala, J.F.; Peña-Ramos, E.A.; Hernández, J.; González-Ríos, H. Antioxidant and Antimicrobial Activity of Agave Angustifolia Extract on Overall Quality and Shelf Life of Pork Patties Stored under Refrigeration. J. Food Sci. Technol. 2018, 55, 4413–4423. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, A.; Akhtar, F.; Yousuf, S.; Xess, I.; Khan, L.A.; Manzoor, N. Fungicidal Activity of Thymol and Carvacrol by Disrupting Ergosterol Biosynthesis and Membrane Integrity against Candida. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 41–50. [Google Scholar] [CrossRef]
- Santos-Zea, L.; Gutierrez-Uribe, J.A.; Benedito, J. Effect of Solvent Composition on Ultrasound-Generated Intensity and Its Influence on the Ultrasonically Assisted Extraction of Bioactives from Agave Bagasse (Agave salmiana). Food Eng. Rev. 2020, 144, 98–107. [Google Scholar] [CrossRef]
- Pereira Da Silva, B.; Valente, A.P.; Paz Parente, J. A New Steroidal Saponin from Agave shrevei. Nat. Prod. Res. 2006, 20, 385–390. [Google Scholar] [CrossRef]
- Monterrosas-Brisson, N.; Arenas Ocampo, M.L.; Jiménez-Ferrer, E.; Jiménez-Aparicio, A.R.; Zamilpa, A.; Gonzalez-Cortazar, M.; Tortoriello, J.; Herrera-Ruiz, M. Anti-Inflammatory Activity of Different Agave Plants and the Compound Cantalasaponin-1. Molecules 2013, 18, 8136–8146. [Google Scholar] [CrossRef]
- Ahumada-Santos, Y.P.; Montes-Avila, J.; de Jesús Uribe-Beltrán, M.; Díaz-Camacho, S.P.; López-Angulo, G.; Vega-Aviña, R.; López-Valenzuela, J.Á.; Heredia, J.B.; Delgado-Vargas, F. Chemical Characterization, Antioxidant and Antibacterial Activities of Six Agave Species from Sinaloa, Mexico. Ind. Crop. Prod. 2013, 49, 143–149. [Google Scholar] [CrossRef]
- Zamora-Gasga, V.; Loarca-Piña, G.; Vázquez-Landaverde, P.A.; Ortiz-Basurto, R.I.; Tovar, J.; Sáyago-Ayerdi, S.G. In vitro colonic fermentation of food ingredients isolated from Agave tequilana Weber var. azul applied on granola bars. LWT Food Sci. Technol. 2015, 60, 766–772. [Google Scholar] [CrossRef]
- Bouaziz, M.A.; Bchir, B.; Chalbi, H.; Sebii, H.; Karra, S.; Smaoui, S.; Attia, H.; Besbes, S. Techno-Functional Characterization and Biological Potential of Agave americana Leaves: Impact on Yoghurt Qualities. J. Food Meas. Charact. 2021, 15, 309–326. [Google Scholar] [CrossRef]
- Santiago-García, P.A.; Mellado-Mojica, E.; León-Martínez, F.M.; López, M.G. Evaluation of Agave angustifolia Fructans as Fat Replacer in the Cookies Manufacture. LWT Food Sci. Technol. 2017, 77, 100–109. [Google Scholar] [CrossRef]
- Santiago-García, P.A.; Mellado-Mojica, E.; León-Martínez, F.M.; Dzul-Cauich, J.G.; López, M.G.; García-Vieyra, M.I. Fructans (agavins) from Agave angustifolia and Agave potatorum as Fat Replacement in Yogurt: Effects on Physicochemical, Rheological, and Sensory Properties. LWT 2021, 140, 110846. [Google Scholar] [CrossRef]
- Ortiz-Basurto, R.I.; Rubio-Ibarra, M.E.; Ragazzo-Sanchez, J.A.; Beristain, C.I.; Jiménez-Fernández, M. Microencapsulation of Eugenia uniflora L. Juice by Spray Drying Using Fructans with Different Degrees of Polymerisation. Carbohydr. Polym. 2017, 175, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Ragazzo-Sánchez, J.A.; Barrón-Carrillo, D.; Sánchez-Burgos, J.A.; Calderón-Santoyo, M.; Montalvo-González, E.; González-Cruz, E.M.; Barros-Castillo, J.C.; de Lourdes García-Magaña, M. Utilization of by-products of endemic fruits: Encapsulation of proteolytic extracts of guamara (Bromelia pinguin) and cocuixtle (Bromelia karatas) by electrospraying. LWT 2021, 149, 111670. [Google Scholar] [CrossRef]
- Ramos-Hernández, J.A.; Lagarón, J.M.; Calderón-Santoyo, M.; Prieto, C.; Ragazzo-Sánchez, J.A. Enhancing hygroscopic stability of agave fructans capsules obtained by electrospraying. J. Food Sci. Technol. 2021, 58, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Rodríguez, A.; Heredia-Olea, E.; Barba-Dávila, B.A.; Gutiérrez-Uribe, J.A.; Antunes-Ricardo, M. Polysaccharides from Agave salmiana Bagasse Improves the Storage Stability and the Cellular Uptake of Indomethacin Nanoemulsions. Food Bioprod. Process. 2021, 127, 114–127. [Google Scholar] [CrossRef]
- Davis, S.C.; Dohleman, F.G.; Long, S.P. The Global Potential for Agave as a Biofuel Feedstock. GCB Bioenergy 2011, 3, 68–78. [Google Scholar] [CrossRef]
- Aguilar, D.L.; Rodríguez-Jasso, R.M.; Zanuso, E.; de Rodríguez, D.J.; Amaya-Delgado, L.; Sanchez, A.; Ruiz, H.A. Scale-up and Evaluation of Hydrothermal Pretreatment in Isothermal and Non-Isothermal Regimen for Bioethanol Production Using Agave Bagasse. Bioresour. Technol. 2018, 263, 112–119. [Google Scholar] [CrossRef]
- Corbin, K.R.; Byrt, C.S.; Bauer, S.; Debolt, S.; Chambers, D.; Holtum, J.A.M.; Karem, G.; Henderson, M.; Lahnstein, J.; Beahan, C.T.; et al. Prospecting for Energy-Rich Renewable Raw Materials: Agave Leaf Case Study. PLoS ONE 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valdez-Vazquez, I.; Alatriste-Mondragón, F.; Arreola-Vargas, J.; Buitrón, G.; Carrillo-Reyes, J.; León-Becerril, E.; Mendez-Acosta, H.O.; Ortíz, I.; Weber, B. A Comparison of Biological, Enzymatic, Chemical and Hydrothermal Pretreatments for Producing Biomethane from Agave Bagasse. Ind. Crop. Prod. 2020, 145, 112160. [Google Scholar] [CrossRef]
- Madhu, P.; Sanjay, M.R.; Jawaid, M.; Siengchin, S.; Khan, A.; Pruncu, C.I. A New Study on Effect of Various Chemical Treatments on Agave americana Fiber for Composite Reinforcement: Physico-Chemical, Thermal, Mechanical and Morphological Properties. Polym. Test. 2020, 85, 106437. [Google Scholar] [CrossRef]
- Leal-Díaz, A.M.; Santos-Zea, L.; Martínez-Escobedo, H.C.; Guajardo-Flores, D.; Gutiérrez-Uribe, J.A.; Serna-Saldivar, S.O. Effect of Agave americana and Agave salmiana Ripeness on Saponin Content from Aguamiel (Agave Sap). J. Agric. Food Chem. 2015, 63, 3924–3930. [Google Scholar] [CrossRef]
- Soto-Castro, D.; Pérez-Herrera, A.; García-Sánchez, E.; Santiago-García, P.A. Identification and Quantification of Bioactive Compounds in Agave potatorum Zucc. Leaves at Different Stages of Development and a Preliminary Biological Assay. Waste Biom. Valoriz. 2021. [Google Scholar] [CrossRef]
- Cruz-Salas, C.N.; Prieto, C.; Calderón-Santoyo, M.; Lagarón, J.M.; Ragazzo-Sánchez, J.A. Micro-and Nanostructures of Agave Fructans to Stabilize Compounds of High Biological Value via Electrohydrodynamic Processing. Nanomaterials 2019, 9, 1659. [Google Scholar] [CrossRef] [Green Version]
- González-Álvarez, M.; Moreno-Limón, S.; Salcedo-Martínez, S.; Pérez-Rodríguez, E. In Vitro Evaluation of Antifungal Activity of Agave (Agave scabra, Salm Dyck) Extracts against Post-Harvest Mushrooms. Phyton (B Aires) 2015, 84, 427–434. [Google Scholar] [CrossRef]
- Ben Hamissa, A.M.; Seffen, M.; Aliakbarian, B.; Casazza, A.A.; Perego, P.; Converti, A. Phenolics Extraction from Agave americana (L.) Leaves Using High-Temperature, High-Pressure Reactor. Food Bioprod. Process. 2012, 90, 17–21. [Google Scholar] [CrossRef]
- Ibarra-Cantún, D.; Ramos-Cassellis, M.E.; Marín-Castro, M.A.; Castelán-Vega, R.D.C. Secondary Metabolites and Antioxidant Activity of the Solid-State Fermentation in Apple (Pirus malus L.) and Agave Mezcalero (Agave angustifolia H.) Bagasse. J. Fungi 2020, 6, 137. [Google Scholar] [CrossRef] [PubMed]
- El-Hawary, S.S.; El-Kammar, H.A.; Farag, M.A.; Saleh, D.O.; El Dine, R.S. Metabolomic Profiling of Five Agave Leaf Taxa via UHPLC/PDA/ESI-MS Inrelation to Their Anti-Inflammatory, Immunomodulatory and Ulceroprotective Activities. Steroids 2020, 160, 108648. [Google Scholar] [CrossRef] [PubMed]
- Monte, J.; Abreu, A.; Borges, A.; Simões, L.; Simões, M. Antimicrobial Activity of Selected Phytochemicals against Escherichia coli and Staphylococcus Aureus and Their Biofilms. Pathogens 2014, 3, 473–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddhapura, S.; Maharshi, A.; Thaker, V. Varietal Difference in Antifungal Activity of Some Species of Agave. Arch Phytopathol. Plant Prot. 2011, 44, 135–141. [Google Scholar] [CrossRef]
- Yan, X.; Corbin, K.R.; Burton, R.A.; Tan, D.K.Y. Agave: A Promising Feedstock for Biofuels in the Water-Energy-Food-Environment (WEFE) Nexus. J. Clean. Prod. 2020, 261, 121283. [Google Scholar] [CrossRef]
- Davis, S.C.; Kuzmick, E.R.; Niechayev, N.; Hunsaker, D.J. Productivity and Water use Efficiency of Agave americana in the First Field Trial as Bioenergy Feedstock on Arid Lands. GCB Bioenergy 2017, 9, 314–325. [Google Scholar] [CrossRef]
- Yang, L.; Lu, M.; Carl, S.; Mayer, J.A.; Cushman, J.C.; Tian, E.; Lin, H. Biomass Characterization of Agave and Opuntia as Potential Biofuel Feedstocks. Biomass Bioenergy 2015, 76, 43–53. [Google Scholar] [CrossRef]
- Núñez, H.M.; Rodríguez, L.F.; Khanna, M. Agave for Tequila and Biofuels: An Economic Assessment and Potential Opportunities. GCB Bioenergy 2011, 3, 43–57. [Google Scholar] [CrossRef]
- Barrera, I.; Amezcua-Allieri, M.A.; Estupiñan, L.; Martínez, T.; Aburto, J. Technical and Economical Evaluation of Bioethanol Production from Lignocellulosic Residues in Mexico: Case of Sugarcane and Blue Agave Bagasses. Chem. Eng. Res. Des. 2016, 107, 91–101. [Google Scholar] [CrossRef]
- Aguilar, D.L.; Rodríguez-Jasso, R.M.; Zanuso, E.; Lara-Flores, A.A.; Aguilar, C.N.; Sanchez, A.; Ruiz, H.A. Operational Strategies for Enzymatic Hydrolysis in a Biorefinery. In Biorefining of Biomass to Biofuels; Springer: Cham, Switzerland, 2018; pp. 223–248. [Google Scholar] [CrossRef]
- Palomo-Briones, R.; López-Gutiérrez, I.; Islas-Lugo, F.; Galindo-Hernández, K.L.; Munguía-Aguilar, D.; Rincón-Pérez, J.A.; Cortés-Carmona, M.Á.; Alatriste-Mondragón, F.; Razo-Flores, E. Agave Bagasse Biorefinery: Processing and Perspectives. Clean Technol. Environ. Policy 2018, 20, 1423–1441. [Google Scholar] [CrossRef]
- Hernández-Hernández, H.M.; Chanona-Pérez, J.J.; Vega, A.; Ligero, P.; Farrera-Rebollo, R.R.; Mendoza-Pérez, J.A.; Calderón-Domínguez, G.; Vera, N.G. Spectroscopic and Microscopic Study of Peroxyformic Pulping of Agave Waste. Microsc. Microanal. 2016, 22, 1084–1097. [Google Scholar] [CrossRef]
- Láinez, M.; Ruiz, H.A.; Castro-Luna, A.A.; Martínez-Hernández, S. Release of Simple Sugars from Lignocellulosic Biomass of Agave salmiana Leaves Subject to Sequential Pretreatment and Enzymatic Saccharification. Biomass Bioenergy 2018, 118, 133–140. [Google Scholar] [CrossRef]
- Maran, J.P.; Priya, B. Ultrasound-Assisted Extraction of Pectin from Sisal Waste. Carbohydr. Polym. 2015, 115, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Brito, J.Q.A.; Dias, F.D.S.; Cunha, S.; Ramos, L.P.; Teixeira, L.S.G. Multiple Response Optimization of Alkaline Pretreatment of Sisal Fiber (Agave sisalana) Assisted by Ultrasound. Biotechnol. Prog. 2019, 35, e2802. [Google Scholar] [CrossRef] [PubMed]
- Ríos-González, L.J.; Medina-Morales, M.A.; Rodríguez-De la Garza, J.A.; Romero-Galarza, A.; Medina, D.D.; Morales-Martínez, T.K. Comparison of Dilute Acid Pretreatment of Agave Assisted by Microwave versus Ultrasound to Enhance Enzymatic Hydrolysis. Bioresour. Technol. 2021, 319, 124099. [Google Scholar] [CrossRef] [PubMed]
- Canales-Flores, R.A.; Prieto-García, F. Taguchi Optimization for Production of Activated Carbon from Phosphoric Acid Impregnated Agricultural Waste by Microwave Heating for the Removal of Methylene Blue. Diam. Relat. Mater. 2020, 109, 108027. [Google Scholar] [CrossRef]
- López-Salazar, H.; Camacho-Díaz, B.H.; Ávila-Reyes, S.V.; Pérez-García, M.D.; González-Cortazar, M.; Arenas Ocampo, M.L.; Jiménez-Aparicio, A.R. Identification and Quantification of β-Sitosterol β-d-Glucoside of an Ethanolic Extract Obtained by Microwave-Assisted Extraction from Agave angustifolia Haw. Molecules 2019, 24, 3926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández, Y.R.; García Serrano, L.A.; Maruri, D.T.; Jiménez Aparicio, A.R.; Camacho Díaz, B.H.; Arenas Ocampo, M.L. Optimization of the Microwave-Assisted Ethanosolv Extraction of Lignocellulosic Compounds from the Bagasse of Agave angustifolia Haw Using the Response Methodology. J. Agric. Food Chem. 2018, 66, 3533–3540. [Google Scholar] [CrossRef]
- Simón, J.; Villanueva-Maldonado, J.; Castillo-Soria, F.R.; Cardenas-Juarez, M.; Briones, E.; Sandoval-Arechiga, R.; Soriano-Equigua, L.; Alvarez-Flores, J.L. Comparison of the Microwave Absorption Properties of Opuntia ficus-indica, Agave atrovirens, and Cocos Nucifera L. Husk. Int. J. Antennas Propag. 2019, 2019, 5872141. [Google Scholar] [CrossRef]
- Navarro, A.; Montiel, C.; Gracia-Fadrique, J.; Tecante, A.; Bárzana, E. Supercritical Carbon Dioxide “Explosion” on Blue Agave Bagasse to Enhance Enzymatic Digestibility. Biomass Convers. Biorefin. 2021. [Google Scholar] [CrossRef]
- Liñán-Montes, A.; De La Parra-Arciniega, S.M.; Garza-González, M.T.; García-Reyes, R.B.; Soto-Regalado, E.; Cerino-Córdova, F.J. Characterization and Thermal Analysis of Agave Bagasse and Malt Spent Grain. J. Therm. Anal. Calorim. 2014, 115, 751–758. [Google Scholar] [CrossRef]

| Component (%) | Agave tequilana | Agave salmiana | Agave durangensis | Agave americana | Agave fourcroydes | Agave angustifolia | Agave tequilana | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Bagasse 1 | Leaves 2 | Bagasse 3 | Leaves 4 | Leaves 5 | Leaves 6 | Stalk 7 | Leaves 8 | Spines 8 | Bagasse 9 | |
| Moisture | 6.44–8.5 | - | - | - | 6.9 | 9.3 | - | - | - | 7.78 |
| Protein | 3.7–3.8 | 6.6–8.35 | 2.5–4.4 | 4.8 | - | - | - | - | - | - |
| Lipids | 0.3 | - | - | - | - | - | - | - | - | - |
| Ashes | 2.0–7.4 | 7.5 | 2.1–6.2 | 7.6 | 16.6 | 3.3 | - | - | - | 1.3 |
| Holocellulose | - | - | - | - | 20.4 | - | - | - | - | - |
| Hemicellulose | 4.4–20 | 15.2 -19.7 | 4.6 | 3.7 | - | 5.6–18.4 | - | 24 | 45 | 34.1 |
| Cellulose | 41.8–42.0 | 38.9–54.5 | 35.0 | 20.7 | - | 65.2–68.5 | - | 72 | 52 | 48.0 |
| Lignin | 7.1–20.1 | 9.8–16.3 | 13.0–19.1 | 9.5–26.1 | 14.5 | 2.7–9.1 | 18 | 14 | 14 | 20.7 |
| Xylan | 13.0–19.9 | 9.5–18.3 | 12.0 | 9.7 | - | - | 13.6 | - | - | - |
| Glucan | 30.9–45.6 | 35.0–38.8 | 34.1 | 35.2 | - | - | - | - | - | - |
| Arabinan | 0.5–0.9 | 1.5–2.1 | 1.0 | 2.4 | - | - | 1.0 | - | - | - |
| Application | By-Product | Compound/Material | Functionality | References |
|---|---|---|---|---|
| Bioactive compound | Leaves, bagasse, and stem (A. Americana, A. salmiana, and A. tequilana) | - Fructans and inulin | - Prebiotics, stimulate microbiota growth. - Enhance lipid homeostasis. - Increase the production of anorectic hormones (GLP-1) and improves the immune response. - Low glycemic index. - Fermentable carbohydrate, stimulate the production of SCFA. - Reduces blood glucose and lipids levels. | [52,53,54,55,56,57,58] |
| Leaves and bagasse (A. americana, A. angustifolia, A. salmiana, A. tequilana) | - Phenolic compounds: Flavonoids (such as glycosylated kaempferol and quercetin, hecogenin, diosgenin, chlorogenin, kammogenin, and gentrogenin) - Phenolic acids: ferulic acid, p-coumaric acid, caffeic acid, p-hydroxybenzoic acid, syringic acid | - Found in the bagasse after pulque and tequila processing. - Identified in leaves of several agave species. - Antioxidant and anti-inflammatory effect when tested by in vitro methods. - Antimicrobial activity. | [26,28,51,52,55,56,59] | |
| Bagasse (A. tequilana, A. salmiana) | - Pyranones and pyrazynes. | - Antioxidant capacity. | [31,59] | |
| Agave extracts | -Terpenes | - Antifungal activity. | [5,60] | |
| Leaves and bagasse (A. salmiana, A. tequilana, A. sisalana, A. attenuata, and A. shevrei) | - Steroidal aponins (Kammogenin glycosides and aglycones, manogenin glycosides) - Sapogenins (hecogenin, thiogenin and canthalasaponin 1). | - Hypocholesterolemic, antiobesity, immunostimulant and parasitic activities. - Anti-inflammatory activity tested in a membrane permeability induced by acetic acid model. - Antimicrobial activity. - Anti-inflammatory and antioxidant activity. | [5,29,30,31,61,62,63,64] | |
| Food ingredient | Residual leaves Cuticle | The whole material | - Roasted or baked for enhanced flavor and color of culinary preparations, boiled to make soup. - Used as wrapping material for barbecue and steamed preparations (mixiote) | [37] |
| Bagasse | Whole bagasse | - Added to oat cookies to improve the fructooligosaccharides content and some techno-functional properties. | [52] | |
| Stem (A. tequilana) | Fructans | - Increased the fiber content and reduced the glycemic index in granola bars. After in vitro fermentation, the production of SCFA was observed. | [56,65] | |
| Leaves (A. americana) | Powder of the whole material | - Used to improve texture, viscosity, and color of steamed yoghurt. | [66] | |
| Fibers (A. angustifolia) | Fructans | - Fat replacer in cookies. Fructans addition lowered the calorie content, increased the soluble fiber, and improved the sensory and techno-functional properties of cookies. | [67,68] | |
| Not specified | Fructans | - Encapsulation of Eugenia uniflora L. with fructans prolonged anthocyanins and color stability and prevented loss of antioxidant capacity. - Encapsulation of proteolytic extracts of Bromelia pinguin and Bromelia karatas with fructans by electrospraying minimized variability of antioxidant capacity during storage. | [69,70] | |
| Not specified | Fructans | - Mixtures of fructans and whey protein were effective to reduce hygroscopicity and to increase thermal stability of Coccoloba uvifera L. leaf extracts. | [71] | |
| Bagasse | Polysaccharides | - Used as encapsulating material for indomethacin nanoemulsions stabilization and dermal uptake. | [72] | |
| Biofuel feedstock | Bagasse (A. tequilana) | Bioethanol | - The bioethanol recovery was 98 and 2% higher than ethanol obtained from corn and sugarcane, respectively. | [73] |
| Bagasse (A. tequilana Weber) | Bioethanol | - Several pretreatments were used to improve saccharification and obtain 87–91% bioethanol yield | [74] | |
| Leaves (A. tequilana) | Bioethanol | - Fermentation of juice extracted from leaves with Saccharomyces cerevisiae rendered 13.8 g/L bioethanol. | [75] | |
| Leaves (A. salmiana) | Bioethanol | - Acid-alkaline pretreatment of leaves was effective to achieve 93% bioethanol yield. | [17] | |
| Bagasse (A. tequilana) | Bioethanol | - Yield of 61–68% bioethanol was reached after 7–13 h fermentation with Saccharomyces cerevisiae. | [18] | |
| Bagasse (A. tequilana) | Hydrogen | - Hydrolysates from bagasse subjected to enzymatic pretreatment and dark digestion, yield 3.4 mol hydrogen/mol. | [24] | |
| Bagasse (A. tequilana) | Hydrogen and methane | - Bagasse pretreated with alkaline hydrogen peroxide increased 1.5 and 3.6 times the hydrogen and methane production, respectively. | [25] | |
| Bagasse (A. tequilana) | Methane | - Anaerobic digestion of the bagasse rendered 0.26 L methane/g COD (chemical oxygen demand). | [23] | |
| Bagasse (A. tequilana) | Methane | - Methane yield was increased 1.5 times by ozone-assisted hydrolysis and dilute acid pretreatment. | [76] | |
| Biomaterials | Fibers (A. americana) | Composites | - Fibers combined with HDPE are suitable for composites with application for roof panels manufacturing. This combination enhanced the tensile strength and thermal stability of composites. | [8] |
| Fibers (A. tequilana) | Reinforcement material for composites | - Agave-polypropylene composites were obtained from heated fibers. Thermal stability did not change, whereas the mechanical properties were negatively affected. | [9] | |
| Fibers (A. tequilana) | Composites | - Agave fiber-filled polypropylene composites improved their mechanical properties and had better compatibility between matrices after fibers addition. | [10] | |
| Bagasse (A. tequilana) | Composites | - Extruded polylactic acid-based composites were added with 20–40% bagasse for plates manufacturing. Higher tensile and flexural strength was achieved with 40 % bagasse. | [12] | |
| Fibers (A. tequilana) | Composites | - Low weight polylactic acid-agave fiber composites were made by rotational molding. However, this method negatively affected the mechanical properties, and produced a material with high porosity. | [13] | |
| Fibers (A. tequilana) | Composites | - The addition of 15–30% agave fiber enhanced the mechanical properties of extruded PHB-bagasse composites and reinforced their toughness. A more thermostable material was produced when a compatibilizer was added (organic peroxide). | [14,15] | |
| Fibers (A. tequilana) | Bioplastic | - Hybrid composites made of pine fiber, agave fiber and HDPE showed higher tensile and flexural strength with 20/80 pine/agave ratio. | [16] | |
| Leaves and bagasse (A. tequilana) | Hybrid composites | - Sonochemical hydrolysis of bagasse and leaves significantly increased the nanocrystals yield. The highest production yield was obtained with leaves (93%). | [40] | |
| Leaves (Agave pulquero) | Nanoparticles | - Alkaline pretreatment of leaves and high-energy planetary micro milling were effective to obtain cellulose nanoparticles (3 nm) with a high degree of crystallinity. | [54] | |
| Leaf fibers (A. americana) | Cellulose nanocrystals | - The chemical pretreatment of fibers improved the composites compatibility with hydrophobic materials and mechanical properties. | [77] |
| By-Product | Process | Application | Main Outcomes | Reference |
|---|---|---|---|---|
| Agave americana leaves | For mercerization, leaves were boiled in 1N NaOH for 24 h at 70 °C. | Mercerization was used to obtain fibers with quality to produce composites. | The alkaline treatment increased tensile strength and interfacial strength of fibers. Thermal stability was also enhanced with this process. | [8] |
| Agave tequilana leaves and bagasse | Pretreatment with sulfuric acid (1–4%) for 30, 60, and 90 min at 115, 120, and 130 °C. Enzymatic saccharification with Cellic® CTec2 at a dose of 3, 6, 10, and 15%. Fermentation was performed with Saccharomyces cerevisiae. | Bioethanol production from bagasse after enzymatic saccharification. | The highest sugar recovery (117.9 mg/g) was obtained with 2% H2SO4/60 min/120 °C with the lowest amount of degradation products, such as acetate and HMF. A maximum saccharification value of 69% and 38.6 g/L bioethanol was achieved. | [18] |
| Agave atrovirens bagasse (metzal and metzontete) | Pretreatment by acid hydrolysis with HCl (1.2% v/v) or combined alkaline pretreatment with NaOH (2%) followed by enzymatic hydrolysis (Viscozyme, Celluclast, Novozyme, Cellubrix and Pulpzyme). Fermentation with Saccharomyces cerevisiae at 30 °C for 48 h. | Delignification and enzymatic saccharification for bioethanol production. | A higher yield of glucose was reached with the alkaline-enzymatic pretreatment of metzal (56%). Although higher bioethanol production was obtained by acid hydrolysis of metzal (29.81%) and mezontete (33.42%). | [19] |
| Agave tequilana bagasse | Cooked and uncooked bagasse were used. Dilute acid pretreatment with HCl 5% w/v at 90 °C for 2 h. Anaerobic digestion was performed in a lab-scale PVC reactor. A loading of 5.8 suspended volatile solids/L from a granular sludge, and 5 g/L COD/L bagasse hydrolysate was used at 32 °C, 2N NaOH. | Acid pretreatment, followed by anerobic digestion to obtain methane. | Uncooked bagasse was more suitable for methane production (0.26 CH4/g COD). Acetate (136 mg/L) and propionate (109 mg/L) were obtained as by-products. | [23] |
| Agave salmiana leaves | Dilute acid treatment. HCl 2.7% v/v | Pretreatment for saccharification of fibers obtained from leaves. | Higher monosaccharides and phenolic compounds yield compared with enzymatic hydrolysis. Formation of hydroxymethylfurfural and furfural. | [24] |
| Agave tequilana bagasse | Pretreatment with 2% (w/v) alkaline hydrogen peroxide (AHP) at 50 °C for 1.5–6 h. Saccharification was performed with Celluclast and Viscozyme at 40 °C, 120 rpm, 12 h. Anaerobic digestion was performed with a granular sludge loading 10 volatile solids/L and 5 bagasse hydrolysate/L. | Oxidative delignification with AHP to enhance methane production. | Higher delignification was achieved at 1.5 h after AHP pretreatment. The sequential saccharification with Celluclast first and then Viscozyme was more effective (35%). The hydrogen production was 215.14 ± 13 L H2/kg of agave bagasse after 64 h and for methane, the yield was 0.20 ± 0.02 L CH4/g COD. | [25] |
| Agave tequilana and Agave salmiana bagasse and leaves | Pretreatment with ammonia 0.5–2 g/dry matter (DM) for bagasse and 1–3 g/DM for leaves. Saccharification was performed with a mixture of Cellic® CTec3 and HTec3 (Novozymes) and Multifect® Pectinase. Fermentation was conducted with S. cerevisiae 424A (LNH-ST) at 30 °C/150 rpm/72 h. | Pretreatment for bio-ethanol production. | A high xylans and glucan conversion (>85%) was observed at the optimized conditions that differed for the agave residues. Leaves produced greater yield in the saccharification process and during fermentation. Up to 19.8% and 15–17% ethanol yield was achieved for leaves and bagasse, respectively. | [26] |
| Agave tequilana bagasse | IL-10% bagasse/[C2mim][OAc]) autoclaved at 120 °C for 3 h. Organosolv–50 g bagasse, 4.5% water, 25% ethanol and 0.5% H2SO4. Processed at 160 °C, 138 psi and 10 min in a high-pressure chemical reactor. Saccharification (50 °C/48 h) with cellulase CTec2® and enxylanase HTec2® (Novozyme), respectively and fermentation (48 h) at 37 °C/60 rpm. | Pretreatment of bagasse to improve saccharification and bioethanol production. | IL–86% xylan removal, 45% lignin reduction. Organosolv–50% xylan removal, 45% lignin reduction. High glucan (>90%) and xylan (>84%) conversion into simple sugars. Ethanol yield was similar, 82% for IL and 85% for organosolv. | [27] |
| Agave tequilana bagasse | The autohydrolysis was carried out in a bioreactor at 140–200 °C for 15–60 min, loaded with 83.33 g bagasse and 500 mL water. Saccharification was performed with Cellic®, then a fermentation with S. cerevisiae was conducted at 32 °C, 100 rpm and 12 h. | A pretreatment using autohydrolysis was optimized to improve saccharification and bioethanol production. | The optimal conditions that increased the bagasse digestibility were: 90 °C, 30 min. The saccharification yield was 74.3% that allowed to obtain 98% yield of bioethanol (65.6 g/L) after 10 h. | [28] |
| Agave atrovirens Karw, fibers from leaves | Peroxyformic treatment: formic acid (80, 87.5, and 95%) hydrogen peroxide (2, 3, and 4%). Processing conditions: 60, 120, and 180 min at 60, 70, and 80 °C. | Organosolv pulping to obtain high purity fibers. | The best yield was obtained with 80% formic acid, 2% hydrogen peroxide, 180 °C for 70 min. A high purity was achieved since very low lignin content was found (k = 3.6). | [94] |
| Agave salmiana leaves | Pretreatment with 1–4% (v/v) sulfuric acid at 121 °C and 30–60 min. A subsequent treatment with 1–4% NaOH under the same conditions. Saccharification was performed with Celluclast at 50 °C, 150 rpm for 48 h. | A sequential acid-alkaline pretreatment was used to improve saccharification. | Delignification (91%) and saccharification (93.1%) was successfully achieved with the sequential pretreatment. The best conditions were 1% sulfuric acid (90 °C) and 3.4% NaOH (70 °C). | [95] |
| Agave tequilana bagasse | Autohydrolysis—1:10 bagasse to water at 180 °C, heated in a pressurized batch reactor (140 psi). AFEX-Anhydrous liquid ammonia was added to bagasse (2:1) and heated in a high-pressure stainless-steel Parr reactor at 102 °C for 30 min. IL-10% bagasse/[C2mim][OAc]) autoclaved at 120 °C for 3 h. Saccharification (50 °C/72 h) with cellulase CTec2 and enxylanase HTec2, (Novozyme). | Comparison between pretreatments of bagasse to reduce recalcitrance and improve saccharification. | Autohydrolysis and IL had the highest lignin and xylan removal. Autohydrolysis: Residual content 5.9% xylan, 34.3% lignin, 14.4 g/L of simple sugars. AFEX: Residual content 15.3% xylan, 18.2% lignin, 21.4 g/L IL: Residual content 14.6% xylan, 13.8% lignin, 25.5 g/L of simple sugars. Untreated bagasse: Residual content 4.1 g/L of simple sugars | [47] |
| Agave tequilana bagasse | IL-15% bagasse/[C2mim][OAc]) autoclaved at 120–160 °C for 3 h. Enzymatic saccharification (55 °C for 72 h) with cellulase CTec2 and enxylanase HTec2, (Novozyme). | Application of IL pretreatment to improve saccharification. | The use of IL at 160 °C allowed 45 % delignification, and saccharification efficiency of 85% (7.64 mg/mL of simple sugars). | [42] |
| Agave tequilana bagasse | IL-10% bagasse/[C2mim][OAc]) autoclaved at 40–160 °C for 3 h. | IL pretreatment for enhanced delignification, morphological and structural properties. | Pretreatment at 120 °C removed the highest amount of lignin, glucans, and xylans. A decrease in cellulose crystallinity was also observed. | [43] |
| Agave durangensis leaves | Ultrasound pretreatment (42 kHz, 132 W, 60 min) or autoclaving at 120 °C for 60 min. Phenolic compounds were analyzed by HPLC. Additionally, inulinase, β-fructofuranosidase and cellulase were produced by submerged fermentation with spores of A. niger isolated from A. durangensis. | US pretreatment for modification of the leaves’ structure and enzyme production. | The US treatment increased the lignin content up to 43% and holocellulose up to 45%, but decreased fructans, fructooligosaccharides, and simple sugars. Moreover, certain phenolics. such as quercetin glucuronide, rutin, and procyanidin B2. The enzymatic activity was also enhanced, specifically the inulase and cellulase activity. | [34] |
| Agave americana biomass (by-product non specified) | Pretreatment with 72% sulfuric acid at 121 °C for 1 h. Glucosidase 49291–1G, cellulase Onozuka R-10, pectinase Guojiaomei, and Cellulase Celluclast were used for saccharification at 50 °C, 150 rpm, 72 h. Lignocellulosic enzymes were produced from spores of Aspergillus niger Gyx086 fermented for 8 days at 30 °C. | Biomass saccharification. | The sugar content after acid pretreatment was 29.1%. The combination of glucosidase and pectinase or pectinase alone showed the highest saccharification activity. Lignocellulosic enzymes had mainly PG and xylanase activity. This allowed to obtain reducing sugars at 35 °C for 72 h. | [44] |
| Agave sisalana fibers (sisal waste) | Ultrasound was used under the following conditions: US power 50–70 W, temperature 40–60 °C, time 10–30 min, and solid-liquid ratio 20–40 g/mL. | US-assisted extraction of pectin from sisal waste. | The best conditions for pectin extraction were 61 W, 50 °C, and 26 min with 30 % yield. | [96] |
| Agave sisalana fibers (sisal waste) | Ultrasound at 400 W and 24 kHz. Sisal fibers and a 1–6% (m/v) NaOH solution, 10–50 min, 30–90% amplitude. | US pretreatment for delignification and cellulose solubilization | The optimal values were 27 min, 4.1% (m/v) NaOH, and 50% amplitude. An 82% removal of hemicellulose and 86% of lignin was achieved. | [97] |
| Agave lechuguilla leaves | Ultrasound were applied at 750 W, 20 kHz, and 60 °C. The microwave treatment was applied at 1000 W. The conditions tested were: 0.5–1.5% (v/v) H2SO4; 5–15 min, 60–140 °C, amplitude 40–100%, solid to liquid ratio 1:12–1:36 (w/v) | Comparison between US and microwave treatment to remove lignin | The H2SO4 concentration was the most important factor that improved the enzymatic hydrolysis. US allowed a higher removal of lignin (67%), whereas microwaving resulted in a lower hemicellulose content and higher sugar content (74%) | [98] |
| Agave sisalana waste | Optimization for saponins extraction was performed with 0–100 ethanol, 30–70 °C, 100–300 rpm, 0.17–0.33 waste/solvent ratio for 4 h. Micellar extraction was conducted with 5% (v/v) Triton-X. | Extraction of bioactive compounds. | Micellar extraction with Triton-X showed a higher saponins recovery than other methods (US and US-assisted micellar extraction). Low yield for phenolic compounds was obtained (22–27%). | [30] |
| Agave salmiana bagasse | Supercritical extraction was carried out at 700 bars and 70 °C. Ultrasound were applied at 60 W and 30 kHz. A mass load of 5 and 10 g was used with pressure 150–450 bar, 40–60 °C, and 5–10% co-solvent. | Ultrasonically-assisted supercritical fluid treatment to extract saponins. | An increase (25%) in the saponin content and antioxidant capacity was observed after US application with the lower mass load. Higher pressure and temperature increased the antioxidant capacity (300 bar and 60 °C with 10% co-solvent). | [31] |
| Agave salmiana bagasse | Control without US application: 60 °C, 58% ethanol, and S/M 20 under mechanical agitation for 60 min. US treatment: 60 °C, 0% ethanol (100% water), and solvent to mass ratio (S/M) of 20. | Steroidal saponins extraction. | Optimal conditions for US-assisted extraction were 60 °C, 0% ethanol (100% water), and S/M 20. The highest saponins recovery was 24.41 mg/g for US and 22.48 for control samples. | [59] |
| Agave salmiana leaves | Leaves were pyrolyzed and activated with H3PO4 (1:2 w/v). To obtain activated carbon (AC), microwaves were applied at 200–600 W, 2–6 min, 30–85% H3PO4, and nitrogen flow rate of 100–200 mL/min. | Microwaves were used as pretreatment to obtain activated carbon for removal of methylene blue. | The optimal conditions were 200 W, 4 min, 60% H3PO4, and nitrogen flow rate of 200 mL/min. AC yield ranged from 73 to 81%, that removed 71.41% of methylene blue. | [99] |
| Agave angustifolia stem | Microwaves were applied at 300 W and 2450 MHz, for 5–15 s. An ethanolic solution of 0.2 n KOH was used for the extraction. | Microwaves were applied for extraction of phytosterols. | The lowest extraction time (5 s) had the highest yield of 124.76 mg b-Sitosterol b-d-Glucoside/g. | [100] |
| Agave angustifolia Haw | Organosolv assisted by microwaves application—40–60% ethanol and 1–2 h, 0.1 % HCl | Organosolv assisted with microwaves for bagasse fractionation and bioetanol production. | The highest extraction yield (70.39%) was achieved at 50% ethanol for 1.5 h. | [101] |
| Agave atrovirens leaves (branches) | A WR90 straight rectangular waveguide was used to measure the absorption coefficient. | The microwaves’ absorption capacity of leaves was measured. | Agave leaves were better microwaves absorbers than other plant materials, being a good alternative to polyurethane, a material commonly used as absorber. | [102] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-Chávez, J.; Villamiel, M.; Santos-Zea, L.; Ramírez-Jiménez, A.K. Agave By-Products: An Overview of Their Nutraceutical Value, Current Applications, and Processing Methods. Polysaccharides 2021, 2, 720-743. https://doi.org/10.3390/polysaccharides2030044
Álvarez-Chávez J, Villamiel M, Santos-Zea L, Ramírez-Jiménez AK. Agave By-Products: An Overview of Their Nutraceutical Value, Current Applications, and Processing Methods. Polysaccharides. 2021; 2(3):720-743. https://doi.org/10.3390/polysaccharides2030044
Chicago/Turabian StyleÁlvarez-Chávez, Jimena, Mar Villamiel, Liliana Santos-Zea, and Aurea K. Ramírez-Jiménez. 2021. "Agave By-Products: An Overview of Their Nutraceutical Value, Current Applications, and Processing Methods" Polysaccharides 2, no. 3: 720-743. https://doi.org/10.3390/polysaccharides2030044