Hydrocarbon Compatible SOFC Anode Catalysts and Their Syntheses: A Review
Abstract
:1. Introduction
2. Utilization of Hydrocarbon Fuels in SOFC
2.1. Carburization
2.1.1. Carburization Mechanism
2.1.2. Carburization Kinetics
- (i)
- Methane cracking
- (ii)
- Reduction of carbon monoxide
- (iii)
- Boudouard reaction
- Nc—parameter to quantify carburization vulnerability
- Dc—diffusivity of carbon in metal at a given temperature
- Xc—maximum solubility of carbon in the metal
- Ac—carbon activity.
2.1.3. Strategies to Control Carburization
3. Kinetics of Electrochemical Oxidation of Fuel at Anode
3.1. Electrochemical Oxidation of H2
3.1.1. Electrocatalytic Activity of Metals
3.1.2. Oxygen Ionic Conductivity of Anode Composite
3.2. Electrochemical Oxidation of Carbon Monoxide
3.3. Electrochemical Oxidation of Hydrocarbon
4. Hydrocarbon (HC) Compatible Anodes
4.1. Ceramic Anodes
4.1.1. Perovskite Structure
Chromites
Titanates
Vanadates
Molybdates
Manganites
Manganates
4.1.2. Fluorite Structure
Ceria-Based Oxides
4.1.3. Other Oxides
4.2. Cermets
4.2.1. Ni-Based Cermet
4.2.2. Cu-Based Cermets
4.2.3. Other Metal-Based Cermets
4.2.4. Ni-Cerate/Zirconate-Based Cermets
4.3. Bimetallic Cermets
4.3.1. Ni–Cu Systems
4.3.2. Ni–Fe System
4.3.3. Co–Cu System
4.3.4. Ni–Co System
4.3.5. Fe–Cu System
4.3.6. Ni–Mo System
5. Long Term Stability of Hydrocarbon Compatible SOFC Anodes
6. H2S Poisoning Issue
6.1. Oxide Anodes
6.2. Cermet Anodes
6.3. Bimetallic Cermets
7. Area Specific Resistance (ASR) of SOFCs Based on Hydrocarbon Compatible Anodes
8. Synthesis of Anode Composites
8.1. Powder Route
8.1.1. Solid-State Method
8.1.2. Wet Chemical Routes
- Solution combustion synthesis
- Sol–gel synthesis and
- Co-precipitation synthesis
- Hydrothermal method.
8.2. In Situ Exsolution Route
8.3. Infiltration
8.3.1. Infiltrated Porous Electrolyte Backbone Electrodes
8.3.2. Infiltrated Mixed Ionic and Electronic Conductors (MIEC) Backbone Electrodes
8.3.3. Composite Backbone Electrodes
8.4. Mechanofusion
8.5. Coating Routes
8.5.1. Electroless Coating
8.5.2. RF-Sputtering
8.5.3. Reverse Current Treatment
- Identical RCT leads to the same performance improvement of ASC cells (Total ASR was reduced by ~10%) between 675 and 725 °C.
- Below 0.005 atm pH2O, the reoxidation process takes place much slower than at 0.05 atm pH2O and does not build up a high-performing nano-structured interlayer.
- Above 0.05 atm pH2O, humidity prevents the ASC cell to reach the required decomposition voltage of the electrolyte material YSZ, and no change in anode performance is detectable.
- Single RCT with a duration of 40 s for the applied reverse current induces thickest and best-performing nano-structured interlayers.
8.5.4. Electrophoretic Deposition
8.5.5. Spray Pyrolysis
9. Nano Anode Composite
9.1. Synthesis
9.2. Bottlenecks
10. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Fuel Cells: A Better Energy Source for Earth and Space. Available online: https://www.nasa.gov/centers/glenn/technology/fuel_cells.html (accessed on 15 October 2021).
- Dicks, A.L.; Rand, D.A.J. Fuel Cell Systems Explained, 3rd ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Yu, H.; Hebling, C. Fuel Cells: Microsystems. In Encyclopedia of Materials: Science and Technology, 2nd ed.; Pergamon: Oxford, UK, 2005; pp. 1–13. [Google Scholar]
- Mendonça, C.; Ferreira, A.; Santos, D.M.F. Towards the Commercialization of Solid Oxide Fuel Cells: Recent Advances in Materials and Integration Strategies. Fuels 2021, 2, 393–419. [Google Scholar] [CrossRef]
- Sammes, N.M.; Bove, R.; Pusz, J. Solid Oxide Fuel Cells. In Fuel Cell Technology: Reaching Towards Commercialization, 1st ed.; Sammes, N.M., Ed.; Springer: London, UK, 2006; pp. 1–26. [Google Scholar]
- Buckingham, J.; Hodge, C.; Hardy, T. Submarine power and propulsion—Application of technology to deliver customer benefit. In Proceedings of the UDT Europe, Glasgow, UK, 10–12 June 2008. [Google Scholar]
- Van Biert, L.; Godjevac, M.; Visser, K.; Aravind, P.V. A review of fuel cell systems for maritime applications. J. Power Sources 2016, 327, 345–364. [Google Scholar] [CrossRef] [Green Version]
- Winkler, W.G.; Henne, R. Fuel cells in aircrafts and synergies. In Proceedings of the International Symposium and Workshop on Fuel Cells and Hydrogen for Aerospace and Maritime Applications, Hamburg, Germany, 16–17 September 2004; pp. 19–44. [Google Scholar]
- Gottmann, M. Solid Oxide Regenerative Fuel Cell for Airplane Power Generation and Storage. Patent No. WO2003094320, 13 November 2003. [Google Scholar]
- Abdalla, A.M.; Hossain, S.; Petra, P.M.I.; Ghasemi, M.; Azad, A.K. Achievements and trends of solid oxide fuel cells in clean energy field: A perspective review. Front. Energy 2020, 14, 359–382. [Google Scholar] [CrossRef]
- Singh, M.; Zappa, D.; Comini, E. Solid oxide fuel cell: Decade of progress, future perspectives and challenges. Int. J. Hydrogen Energy 2021, 46, 27643–27674. [Google Scholar] [CrossRef]
- Dewa, M.; Yu, W.; Dale, N.; Hussain, A.M.; Norton, M.G.; Ha, S. Recent progress in integration of reforming catalyst on metal-supported SOFC for hydrocarbon and logistic fuels. Int. J. Hydrogen Energy 2021, 46, 33523–33540. [Google Scholar] [CrossRef]
- Wei, T.; Liu, B.; Jia, L.; Li, R. Perovskite materials for highly efficient catalytic CH4 fuel reforming in solid oxide fuel cell. Int. J. Hydrogen Energy 2021, 46, 24441–24460. [Google Scholar] [CrossRef]
- Liu, F.; Duan, C. Direct-Hydrocarbon Proton Conducting Solid Oxide Fuel Cells. Sustainability 2021, 13, 4736. [Google Scholar] [CrossRef]
- Shabri, H.A.; Othman, M.H.D.; Mohamed, M.A.; Kurniawan, T.A.; Jamil, S.M. Recent progress in metal-ceramic anode of solid oxide fuel cell for direct hydrocarbon fuel utilization: A review. Fuel Process. Technol. 2021, 212, 106626. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, Z.; Jin, Y.; Liu, C.; Lei, Z.; Chen, F.; Peng, S. Progress report on the catalyst layers for hydrocarbon-fueled SOFCs. Int. J Hydrog. Energy 2021, 46, 39369–39386. [Google Scholar] [CrossRef]
- Su, H.; Hu, Y.H. Progress in low-temperature solid oxide fuel cells with hydrocarbon fuels. Chem. Eng. J. 2020, 402, 126235. [Google Scholar] [CrossRef]
- Shi, N.; Xie, Y.; Yang, Y.; Xue, S.; Li, X.; Zhu, K.; Huan, D.; Peng, R.; Xia, C.; Lu, Y. Review of anodic reactions in hydrocarbon fueled solid oxide fuel cells and strategies to improve anode performance and stability. Mater Renew. Sustain. Energy 2020, 9, 6. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.A. Kinetics, Catalysis and Mechanism of Methane Steam Reforming. Master’s Thesis, Worcester Polytechnic Institute, Worcester, MA, USA, 2006. [Google Scholar]
- Kalamaras, C.M.; Efstathiou, A.M. Hydrogen production technologies: Current state and future developments. Conf. Pap. Energy 2013, 2013, 690627. [Google Scholar] [CrossRef] [Green Version]
- Chun, C.M.; Ramanarayanan, T.A. Mechanism and Control of Carbon Deposition on High Temperature Alloys. J. Electrochem. Soc. 2007, 154, C465–C471. [Google Scholar] [CrossRef] [Green Version]
- Gross, M.D.; Vohs, J.M.; Gorte, R.J. Recent progress in SOFC anodes for direct utilization of hydrocarbons. J. Mater. Chem. 2007, 17, 3071–3077. [Google Scholar] [CrossRef] [Green Version]
- An, S.; Lu, C.; Worrell, W.L.; Gorte, R.J.; Vohs, J.M. Characterization of Cu–CeO2 direct hydrocarbon anodes in a solid oxide fuel cell with lanthanum gallate electrolyte. Solid State Ion. 2004, 175, 135–138. [Google Scholar] [CrossRef]
- Chen, T.; Wang, W.G.; Miao, H.; Li, T.; Xu, C. Evaluation of carbon deposition behavior on the nickel/yttrium-stabilized zirconia anode-supported fuel cell fueled with simulated syngas. J. Power Sources 2011, 196, 2461–2468. [Google Scholar] [CrossRef]
- Sumi, H.; Lee, Y.-H.; Muroyama, H.; Matsui, T.; Kamijo, M.; Mimuro, S.; Yamanaka, M.; Nakajima, Y.; Eguchi, K. Effect of carbon deposition by carbon monoxide disproportionation on electrochemical characteristics at low temperature operation for solid oxide fuel cells. J. Power Sources 2011, 196, 4451–4457. [Google Scholar] [CrossRef]
- Świrk, K.; Galvez, M.; Motak, M.; Grzybek, T.; Rønning, M.; Da Costa, P. Yttrium promoted Ni-based double-layered hydroxides for dry methane reforming. J. CO2 Util. 2018, 27, 247–258. [Google Scholar] [CrossRef]
- Dean, S.W., Jr. Estimating Metal Dusting Attack on Stainless Steel Alloys in Syngas Environments. In Proceedings of the Corrosion 2001, Houston, TX, USA, 11–16 March 2001; p. 01384. [Google Scholar]
- Gunji, A.; Wen, C.; Otomo, J.; Kobayashi, T.; Ukai, K.; Mizutani, Y.; Takahashi, H. Carbon deposition behaviour on Ni–ScSZ anodes for internal reforming solid oxide fuel cells. J. Power Sources 2004, 131, 285–288. [Google Scholar] [CrossRef]
- Vernoux, P.; Guillodo, M.; Fouletier, J.; Hammou, A. Alternative anode material for gradual methane reforming in solid oxide fuel cells. Solid State Ion. 2000, 135, 425–431. [Google Scholar] [CrossRef]
- Toebes, M.L.; Bitter, J.H.; Van Dillen, A.J.; de Jong, K.P. Impact of the structure and reactivity of nickel particles on the catalytic growth of carbon nanofibers. Catal. Today. 2002, 76, 33–42. [Google Scholar] [CrossRef]
- Baker, R.T.K.; Chludzinski, J.J., Jr. In-situ electron microscopy studies of the behavior of supported ruthenium particles. 2. Carbon deposition from catalyzed decomposition of acetylene. J. Phys. Chem. 1986, 90, 4734–4738. [Google Scholar] [CrossRef]
- Kim, T.; Liu, G.; Boaro, M.; Lee, S.-I.; Vohs, J.M.; Gorte, R.J.; Al-Madhi, O.H.; Dabbousi, B.O. A study of carbon formation and prevention in hydrocarbon-fueled SOFC. J. Power Sources 2006, 155, 231–238. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.; Lu, C.; He, H.; Ahn, K.; Gorte, R.J.; Vohs, J.M. Influence of composition and Cu impregnation method on the performance of Cu/CeO2/YSZ SOFC anodes. J. Power Sources 2006, 154, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.; Li, S.; Zhao, W.; Song, X.; An, S. Study on diffusion of copper of SOFC Cu-based anodes in co-sintering with electrolyte. Adv. Mater. Res. 2012, 415–417, 1647–1650. [Google Scholar] [CrossRef]
- Atkinson, A.; Barnett, S.; Gorte, R.J.; Irvine, J.T.S.; McEvoy, J.; Mogensen, M.; Singhal, S.C.; Vohs, J. Advanced anodes for high-temperature fuel cells. Nat. Mater. 2004, 3, 17–27. [Google Scholar] [CrossRef]
- Rossmeisl, J.; Bessler, W.G. Trends in catalytic activity for SOFC anode materials. Solid State Ion. 2008, 178, 1694–1700. [Google Scholar] [CrossRef]
- Ingram, B.; Linic, S. First-Principles Analysis of the Activity of Transition and Noble Metals in the Direct Utilization of Hydrocarbon Fuels at Solid Oxide Fuel Cell Operating Conditions. J. Electrochem. Soc. 2009, 156, B1457–B1465. [Google Scholar] [CrossRef]
- Ihara, M.; Kusano, T.; Yokoyama, C. Competitive adsorption reaction mechanism of Ni/yttria-stabilized zirconia cermet anodes in H2-H2O solid oxide fuel cells. J. Electrochem. Soc. 2001, 148, A209–A219. [Google Scholar] [CrossRef]
- Vogler, M. Elementary Kinetic Modelling Applied To Solid Oxide Fuel Cell Pattern Anode and a Direct Flame Fule Cell System. Ph.D. Thesis, Ruperto-Carola University of Heidelberg, Heidelberg, Germany, 2009. [Google Scholar]
- Adler, S.B.; Bessler, W.G. Elementary Kinetic Modeling of Solid Oxide Fuel Cell Electrode Reactions; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Lykhnytskyi, K.V.; Barsukov, V.Z.; Jaskuła, M. Promising catalysts for H2-O2 fuel cells (review). In Hydrogen Materials Science and Chemistry of Carbon Nanomaterials; Springer: Dordrecht, The Netherlands, 2007; pp. 177–185. [Google Scholar]
- Virkar, A.V.; Chen, J.; Tanner, C.W.; Kim, J.-W. The role of electrode microstructure on activation and concentration polarizations in solid oxide fuel cells. Solid State Ion. 2000, 131, 189–198. [Google Scholar] [CrossRef]
- Weiss, S.E. Catalysts and Materials Development for Fuel Cell Power Generation. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2005. [Google Scholar]
- An, W.; Gatewood, D.; Dunlap, B.; Heath Turner, C. Catalytic activity of bimetallic nickel alloys for solid-oxide fuel cell anode reactions from density-functional theory. J. Power Sources 2011, 196, 4724–4728. [Google Scholar] [CrossRef]
- Fei, J.; Hou, Z.; Zheng, X.; Yashima, T. Doped Ni catalysts for methane reforming with CO2. Catal. Lett. 2004, 98, 241–246. [Google Scholar] [CrossRef]
- Jiang, Y.; Virkar, A.V. Fuel composition and diluent effect on gas transport and performance of anode-supported SOFCs. J. Electrochem. Soc. 2003, 150, A942–A951. [Google Scholar] [CrossRef]
- Etsell, T.H.; Flengas, S.N. Overpotential behavior of stabilized zirconia solid electrolyte fuel cells. J. Electrochem. Soc. 1971, 118, 1890–1900. [Google Scholar] [CrossRef]
- Karpachov, S.V.; Filyayev, A.T.; Palguyev, S.F. Polarization of carbon monoxide electrodes on platinum in a solid zirconialime electrolyte. Electrochim. Acta 1964, 9, 1681–1685. [Google Scholar] [CrossRef]
- Yakabe, H.; Hishinuma, M.; Uratani, M.; Matsuzaki, Y.; Yasuda, I. Evaluation and modeling of performance of anode-supported solid oxide fuel cell. J. Power Sources 2000, 86, 423–431. [Google Scholar] [CrossRef]
- Holtappels, P.; De Haart, L.G.J.; Stimming, U.; Vinke, I.C.; Mogensen, M. Reaction of CO/CO2 gas mixtures on Ni–YSZ cermet electrodes. J. Appl. Electrochem. 1999, 29, 561–568. [Google Scholar] [CrossRef]
- Yurkiv, V.; Starukhin, D.; Volpp, H.-R.; Bessler, W.G. Elementary reaction kinetics of the CO∕ CO2∕ Ni∕ YSZ electrode. J. Electrochem. Soc. 2011, 158, B5–B10. [Google Scholar] [CrossRef]
- Lauvstad, G.O.; Tunold, R.; Sunde, S. Electrochemical oxidation of CO on Pt and Ni point electrodes in contact with an yttria-stabilized zirconia electrolyte I. Modeling of steady-state and impedance behavior. J. Electrochem. Soc. 2002, 149, E497–E505. [Google Scholar] [CrossRef]
- Lauvstad, G.O.; Tunold, R.; Sunde, S. Electrochemical oxidation of CO on Pt and Ni point electrodes in contact with an yttria-stabilized zirconia electrolyte II. Steady-State and Impedance Measurements. J. Electrochem. Soc. 2002, 149, E506–E514. [Google Scholar] [CrossRef]
- Homel, M.; Gür, T.M.; Koh, J.H.; Virkar, A.V. Carbon monoxide-fueled solid oxide fuel cell. J. Power Sources 2010, 195, 6367–6372. [Google Scholar] [CrossRef]
- Bradford Vannice, M.C.J. CO2 reforming of CH4. Catal. Rev. 1999, 41, 1–42. [Google Scholar] [CrossRef]
- Xu, J.; Froment, G.F. Methane steam reforming, methanation and water-gas shift: I. Intrinsic kinetics. AIChE J. 1989, 35, 88–96. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R. Catalytic Steam Reforming. In Catalysis (Science and Technology); Anderson, J.R., Boudart, M., Eds.; Springer: Berlin/Heidelberg, Germany, 1984; p. 5. [Google Scholar]
- Chen, D.; Lødeng, R.; Anundskås, A.; Olsvik, O.; Holmen, A. Deactivation during carbon dioxide reforming of methane over Ni catalyst: Microkinetic analysis. Chem. Eng. Sci. 2001, 56, 1371–1379. [Google Scholar] [CrossRef]
- Aparicio, L.M. Transient isotopic studies and microkinetic modeling of methane reforming over nickel catalysts. J. Catal. 1997, 165, 262–274. [Google Scholar] [CrossRef]
- Wei, J.; Iglesia, E. Isotopic and kinetic assessment of the mechanism of reactions of CH 4 with CO2 or H2O to form synthesis gas and carbon on nickel catalysts. J. Catal. 2004, 224, 370–383. [Google Scholar] [CrossRef]
- Zhu, J.; van Ommen, J.G.; Lefferts, L. Reaction scheme of partial oxidation of methane to synthesis gas over yttrium-stabilized zirconia. J. Catal. 2004, 225, 388–397. [Google Scholar] [CrossRef]
- Mogensen, M.; Kammer, K. Conversion of hydrocarbons in solid oxide fuel cells. Annu. Rev. Mater. Res. 2003, 33, 321–331. [Google Scholar] [CrossRef]
- Hecht, E.S.; Gupta, G.K.; Zhu, H.; Dean, A.M.; Kee, R.J.; Maier, L.; Deutschmann, O. Methane reforming kinetics within a Ni–YSZ SOFC anode support. Appl. Catal. A Gen. 2005, 295, 40–51. [Google Scholar] [CrossRef]
- Laosiripojana, N.; Assabumrungrat, S. Catalytic steam reforming of methane, methanol, and ethanol over Ni/YSZ: The possible use of these fuels in internal reforming SOFC. J. Power Source 2007, 163, 943–951. [Google Scholar] [CrossRef]
- Alstrup, I.; Tavares, M.T. The kinetics of carbon formation from CH4+ H2 on a silica-supported nickel catalysis. J. Catal. 1992, 135, 147–155. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Zhan, Z.; Liu, J.; Barnett, S.A. Direct operation of solid oxide fuel cells with methane fuel. Solid State Ion. 2005, 176, 1827–1835. [Google Scholar] [CrossRef]
- Steele, B.C.H.; Kelly, I.; Middleton, H.; Rudkin, R. Oxidation of methane in solid state electrochemical reactors. Solid State Ion. 1988, 28, 1547–1552. [Google Scholar] [CrossRef]
- Steele, B.C.H.; Middleton, P.H.; Rudkin, R.A. Material science aspects of SOFC technology with special reference to anode development. Solid State Ion. 1990, 40, 388–393. [Google Scholar] [CrossRef]
- Zhang, M.; Jeerh, G.; Zou, P.; Lan, R.; Wang, M.; Wang, H.; Tao, S. Recent development of perovskite oxide-based electrocatalysts and their applications in low to intermediate temperature electrochemical devices. Mater. Today 2021. [Google Scholar] [CrossRef]
- Wei, Y.; Weng, Z.; Guo, L.; An, L.; Yin, J.; Sun, S.; Da, P.; Wang, R.; Xi, P.; Yan, C.-H. Activation strategies of perovskite-type structure for applications in oxygen-related electrocatalysts. Small Methods 2021, 20215, 2100012. [Google Scholar] [CrossRef]
- Sfeir, J.; Buffat, P.A.; Pedro, M.; Xanthopoulos, N.; Vasquez, R.; Mathieu, H.J.; Van, J.; Thampi, K.R. Lanthanum chromite based catalysts for oxidation of methane directly on SOFC anodes. J. Catal. 2001, 244, 229–244. [Google Scholar] [CrossRef]
- Barison, S.; Battagliarin, M.; Daolio, S.; Fabrizio, M.; Miorin, E.; Antonucci, P.L. Novel Au/La1−xSrx MnO3 and Au / La1− xSrxCrO3 composites: Catalytic activity for propane partial oxidation and reforming. Solid State Ion. 2007, 177, 3473–3484. [Google Scholar] [CrossRef]
- Jardiel, T.; Caldes, M.T.; Moser, F.; Hamon, J.; Gauthier, G.; Joubert, O. New SOFC electrode materials: The Ni-substituted LSCM-based compounds (La0.75Sr0.25)(Cr0.5Mn0.5− xNix)O3− δ and (La0.75Sr0.25)(Cr0.5− xNixMn0.5)O3− δ. Solid State Ion. 2010, 181, 894–901. [Google Scholar] [CrossRef]
- Danilovic, N.; Vincent, A.; Luo, J.; Chuang, K.T.; Hui, R.; Sanger, A.R. Correlation of fuel cell anode electrocatalytic and ex situ catalytic perovskites La0.75Sr0.25Cr0.5X0.5O3−δ (X = Ti, Mn, Fe, Co). Chem. Mater. 2010, 4, 957–965. [Google Scholar] [CrossRef]
- Kobsiriphat, W.; Madsen, B.D.; Wang, Y.; Shah, M.; Marks, L.D.; Barnett, S.A. Nickel- and ruthenium-doped lanthanum chromite anodes: Effects of nanoscale metal precipitation on solid oxide fuel cell performance. J. Electrochem. Soc. 2010, 157, B279–B284. [Google Scholar] [CrossRef]
- Kobsiriphat, W.; Madsen, B.D.; Wang, Y.; Marks, L.D.; Barnett, S.A. La0.8Sr0.2Cr1−x RuxO3−δ–Gd0.1Ce0.9O1.95 solid oxide fuel cell anodes: Ru precipitation and electrochemical performance. Solid State Ion. 2009, 180, 257–264. [Google Scholar] [CrossRef]
- Sauvet, A.; Fouletier, J. Electrochemical properties of a new type of anode material La1−xSrxCr1−yRuyO3−δ for SOFC under hydrogen and methane at intermediate temperatures. Electrochim. Acta. 2001, 47, 987–995. [Google Scholar] [CrossRef]
- Sauvet, A.L.; Fouletier, J.; Gaillard, F.; Primet, M. Surface properties and physicochemical characterizations of a new type of anode material, La1−xSrxCr1−yRuyO3−δ, for a solid oxide fuel cell under methane at intermediate temperature. J. Catal. 2002, 209, 25–34. [Google Scholar] [CrossRef]
- Sauvet, A.L.; Irvine, J.T.S. Catalytic activity for steam methane reforming and physical characterisation of La1−xSr xCr1−yNiyO3−d. Solid State Ion. 2004, 167, 1–8. [Google Scholar] [CrossRef]
- Sun, Y.F.; Li, J.H.; Zeng, Y.M.; Amirkhiz, B.S.; Wang, M.N.; Behnamian, Y.; Luo, J.L. A-site deficient perovskite: The parent for in situ exsolution of highly active regenerable nanoparticles as SOFC anodes. J. Mater Chem. 2015, 3, 11048–11056. [Google Scholar] [CrossRef]
- Sinha, A.; Miller, D.N.; Irvine, J.T.S. Development of novel anode material for intermediate temperature SOFC (IT-SOFC). J. Mater. Chem. 2016, 4, 11117–11123. [Google Scholar] [CrossRef] [Green Version]
- Miller, D.N.; Irvine, J.T.S. B-site doping of lanthanum strontium titanate for solid oxide fuel cell anodes. J. Power Sources 2011, 196, 7323–7327. [Google Scholar] [CrossRef]
- Li, X.; Zhao, H.; Gao, F.; Chen, N.; Xu, N. La and Sc co-doped SrTiO3 as novel anode materials for solid oxide fuel cells. Electrochem. Commun. 2008, 10, 1567–1570. [Google Scholar] [CrossRef]
- Li, X.; Zhao, H.; Xu, N.; Zhou, X.; Zhang, C.; Chen, N. Electrical conduction behavior of La, Co co-doped SrTiO3 perovskite as anode material for solid oxide fuel cells. Int. J. Hydrog. Energ. 2009, 34, 6407–6414. [Google Scholar] [CrossRef]
- Li, X.; Zhao, H.; Gao, F.; Zhu, Z.; Chen, N.; Shen, W. Synthesis and electrical properties of Co-doped Y0.08Sr0.92TiO3−δ as a potential SOFC anode. Solid State Ion. 2008, 179, 1588–1592. [Google Scholar] [CrossRef]
- Périllat-Merceroz, C.; Roussel, P.; Vannier, R.; Gélin, P.; Rosini, S.; Gauthier, G. Lamellar titanates: A breakthrough in the search for new solid oxide fuel cell anode materials operating on methane. Adv. Energy Mater. 2011, 5, 573–576. [Google Scholar] [CrossRef]
- Ovalle, A.; Ruiz-Morales, J.C.; Canales-Vázquez, J.; Marrero-López, D.; Irvine, J.T.S. Mn-substituted titanates as efficient anodes for direct methane SOFCs. Solid State Ion. 2006, 177, 1997–2003. [Google Scholar] [CrossRef]
- Ruiz-Morales, J.C.; Canales-Vázquez, J.; Savaniu, C.; Marrero-López, D.; Núñez, P.; Zhou, W.; Irvine, J.T.S. A new anode for solid oxide fuel cells with enhanced OCV under methane operation. Phys. Chem. Chem. Phys. 2007, 9, 1821–1830. [Google Scholar] [CrossRef]
- Kim, J.H.; Miller, D.; Schlegl, H.; Mcgrouther, D.; Irvine, J.T.S. Investigation of microstructural and electrochemical properties of impregnated (La, Sr)(Ti, Mn)O3−δ as a potential anode material in high-temperature solid oxide fuel cells. Chem. Mater. 2011, 3, 3841–3847. [Google Scholar] [CrossRef]
- Ma, Q.; Tietz, F.; Stöver, D. Nonstoichiometric Y-substituted SrTiO3 materials as anodes for solid oxide fuel cells. Solid State Ion. 2011, 192, 535–539. [Google Scholar] [CrossRef]
- Senthil Kumar, S. Studies on Internal Reforming Solid Oxide Fuel Cell. Ph.D. Thesis, Indian Institute of Science, Bangalore, India, 2018. [Google Scholar]
- Ma, Q.; Tietz, F.; Leonide, A.; Ivers-Tiffée, E. Electrochemical performances of solid oxide fuel cells based on Y-substituted SrTiO3 ceramic anode materials. J. Power Sources 2011, 196, 7308–7312. [Google Scholar] [CrossRef]
- Fu, Q.; Tietz, F.; Sebold, D.; Tao, S.; Irvine, J.T.S. An efficient ceramic-based anode for solid oxide fuel cells. J. Power Sources 2007, 171, 663–669. [Google Scholar] [CrossRef]
- Savaniu, C.D.; Irvine, J.T.S. La-doped SrTiO3 as anode material for IT-SOFC. Solid State Ion. 2011, 192, 491–493. [Google Scholar] [CrossRef]
- Smith, B.H.; Holler, W.C.; Gross, M.D. Electrical properties and redox stability of tantalum-doped strontium titanate for SOFC anodes. Solid State Ion. 2011, 192, 383–386. [Google Scholar] [CrossRef]
- Miao, H.; Chen, B.; Wu, X.; Wang, Q.; Lin, P.; Wang, J.; Yang, C.; Zhang, H.; Yuan, J. Optimizing strontium titanate anode in solid oxide fuel cells by ytterbium doping. Int. J. Hydrogen Energy 2019, 44, 13728–13736. [Google Scholar] [CrossRef]
- Cao, Z.; Fan, L.; Zhang, G.; Shao, K.; He, C.; Zhang, Q.; Lv, Z.; Zhu, B. Titanium-substituted ferrite perovskite: An excellent sulfur and coking tolerant anode catalyst for SOFCs. Catalysts 2019, 330, 217–221. [Google Scholar] [CrossRef]
- Błaszczak, P.; Łapiński, M.; Wang, S.-F.; Jasiński, P.; Bochentyn, B. Exsolution of Ni nanoparticles on the surface of cerium and nickel co-doped lanthanum strontium titanate as a new anodic layer for DIR-SOFC. Anti-coking potential and H2S poisoning resistance of the prepared material. Int. J. Hydrogen Energy 2020, 45, 29186–29200. [Google Scholar] [CrossRef]
- Arrivé, C.; Delahaye, T.; Joubert, O.; Gauthier, G.H. Study of (La,Sr)(Ti,Ni)O3-δ materials for symmetrical solid oxide cell electrode—Part C: Electrical and electrochemical behaviour. Ceram. Int. 2020, 46, 2342–2345. [Google Scholar] [CrossRef]
- Arrivé, C.; Delahaye, T.; Joubert, O.; Gauthier, G.H. Study of (La,Sr)(Ti,Ni)O3-δ materials for symmetrical Solid Oxide Cell electrode—Part B: Conditions of Ni exsolution, Ceram. Int. 2020, 46, 5841–5849. [Google Scholar] [CrossRef]
- Liang, C.; Yang, C.; Wang, J.; Lin, P.; Li, X.; Wu, X.; Yuan, J. Sintering process and effects on LST and LST-GDC particles simulated by molecular dynamics modeling method. Energies 2020, 13, 4128. [Google Scholar] [CrossRef]
- Kim, H.S.; Jeon, Y.; Kim, J.H.; Jang, G.Y.; Yoon, S.P.; Yun, J.W. Characteristics of Sr1−xYxTi1−yRuyO3+/−δ and Ru-impregnated Sr1−xYxTiO3+/−δ perovskite catalysts as SOFC anode for methane dry reforming, Appl. Surf. Sci. 2020, 510, 145450. [Google Scholar] [CrossRef]
- Fung, K.-Z.; Tsai, S.-Y.; Liu, C.-Y. Synthesis and properties of perovskite anode for SOFC applications. ECS Trans. 2013, 57, 1423–1428. [Google Scholar] [CrossRef]
- Macías, J.; Yaremchenko, A.A.; Frade, J.R. Enhanced stability of perovskite-like SrVO3-based anode materials by donor-type substitutions. J. Mater. Chem. A 2016, 4, 10186–10194. [Google Scholar] [CrossRef]
- Adijanto, L.; Padmanabhan, V.B.; Gorte, R.J.; Vohs, J.M. Polarization-induced hysteresis in CuCo-doped rare earth vanadates SOFC anodes. J. Electrochem. Soc. 2012, 159, F751–F756. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhou, Q.; He, Q.; He, T. Double-perovskites A2FeMoO6-δ (A = Ca, Sr, Ba) as anodes for solid oxide fuel cells. J Power Sources 2010, 195, 6356–6366. [Google Scholar] [CrossRef]
- Park, J.; Hasson, I.D.; Gross, M.D.; Chen, C.; Vohs, J.M.; Gorte, R.J. A high-performance solid oxide fuel cell anode based on lanthanum strontium vanadate. J. Power Sources 2011, 196, 7488–7494. [Google Scholar] [CrossRef]
- Cheng, Z.; Zha, S.; Aguilar, L.; Wang, D.; Winnick, J.; Liu, M. A solid oxide fuel cell running on H2S/CH4 fuel mixtures. Electrochem. Solid-State Lett. 2006, 9, A31–A33. [Google Scholar] [CrossRef]
- Cheng, Z.; Zha, S.; Aguilar, L.; Liu, M. Chemical, electrical, and thermal properties of strontium doped lanthanum vanadate. Solid State Ion. 2005, 176, 1921–1928. [Google Scholar] [CrossRef]
- Smith, B.H.; Gross, M.D. A highly conductive oxide anode for solid oxide fuel cells. Electrochem. Solid-State Lett. 2011, 14, B1–B5. [Google Scholar] [CrossRef]
- Falcón, H.; Barbero, J.A.; Araujo, G.; Casais, M.T.; Mart, M.J.; Alonso, J.A.; Fierro, J.L.G. Double perovskite oxides A2FeMoO6 − δ (A = Ca, Sr and Ba) as catalysts for methane combustion. Appl. Catal. B Environ. 2004, 53, 37–45. [Google Scholar] [CrossRef]
- Li, H.J.; Tian, Y.; Wang, Z.M.; Qie, F.C.; Li, Y.D. An all perovskite direct methanol solid oxide fuel cell with high resistance to carbon formation at the anode. RSC Adv. 2012, 2, 3857–3863. [Google Scholar] [CrossRef]
- Yang, X.; Chen, J.; Panthi, D.; Niu, B.; Lei, L.; Yuan, Z.; Du, Y.; Li, Y.; Chen, F.; He, T. Electron doping of Sr2FeMoO6-δ as high performance anode materials for solid oxide fuel cells. J. Mater. Chem. A 2019, 7, 733–743. [Google Scholar] [CrossRef]
- Wang, Y.; Li, P.; Li, H.; Zhao, Y.; Li, Y. Synthesis and enhanced electrochemical performance of Sm-doped Sr2Fe1.5Mo0.5O6. Fuel Cells 2014, 14, 973–978. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Dass, R.I.; Xing, Z.-L.; Goodenough, J.B. Double perovskites as anode materials for solid-oxide fuel cells. Science 2006, 312, 254–257. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Dass, R.I.; Denyszyn, J.C.; Goodenough, J.B. Synthesis and characterization of Sr2MgMoO6-δ—An anode material for the solid oxide fuel cell. J. Electrochem. Soc. 2006, 153, A1266–A1272. [Google Scholar] [CrossRef]
- Li, C.; Wang, W.; Zhao, N.; Liu, Y.; He, B.; Hu, F.; Chen, C. Structure properties and catalytic performance in methane combustion of double perovskites Sr2Mg1−xMnxMoO6 −δ. Appl. Catal. B Environ. 2011, 102, 78–84. [Google Scholar] [CrossRef]
- Marrero-López, D.; Pena-Martinez, J.; Ruiz-Morales, J.C.; Pérez-Coll, D.; Aranda, M.A.G.; Nunez, P. Synthesis, phase stability and electrical conductivity of Sr2MgMoO6− δ anode. Mater. Res. Bull. 2008, 43, 2441–2450. [Google Scholar] [CrossRef]
- Marrero-López, D.; Peña-Martínez, J.; Ruiz-Morales, J.C.; Gabás, M.; Núñez, P.; Aranda, M.A.G.; Ramos-Barrado, J.R. Redox behaviour, chemical compatibility and electrochemical performance of Sr2MgMoO6− δ as SOFC anode. Solid State Ion. 2010, 180, 1672–1682. [Google Scholar] [CrossRef]
- Skutina, L.S.; Vylkov, A.I.; Bainov, I.N.; Chistyakov, K.A.; Kuznetsov, D.K.; Pavlenko, O.B.; Medvedev, D.A. Catalytic properties of Sr2Ni0.75Mg0.25MoO6–δ based composites for application in hydrocarbon-fuelled solid oxide fuel cells. Int. J. Hydrogen Energy 2021, 46, 16899–16906. [Google Scholar] [CrossRef]
- Graves, C.; Sudireddy, B.R.; Mogensen, M. Molybdate based ceramic negative-electrode materials for solid oxide cells. ECS Trans. 2010, 28, 173–192. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Liu, J.; Chen, F.; Du, Y.; Deibel, A.; He, T. Molybdenum-based double perovskites A2CrMoO6−δ (A = Ca, Sr, Ba) as anode materials for solid oxide fuel cells. Electrochim. Acta 2018, 290, 440–450. [Google Scholar] [CrossRef]
- Filonova, E.A.; Dmitriev, A.S.; Pikalov, P.S.; Medvedev, D.A.; Pikalova, E.Y. The structural and electrical properties of Sr2Ni0.75Mg0.25MoO6 and its compatibility with solid state electrolytes. Solid State Ion. 2014, 262, 365–369. [Google Scholar] [CrossRef]
- Dos Santos-Gómez, L.; León-Reina, L.; Porras-Vázquez, J.M.; Losilla, E.R.; Marrero-López, D. Chemical stability and compatibility of double perovskite anode materials for SOFCs. Solid State Ion. 2013, 239, 1–7. [Google Scholar] [CrossRef]
- Osinkin, D.A.; Zabolotskaya, E.V.; Kellerman, D.G.; Suntsov, A.Y. The physical properties and electrochemical performance of Ca-doped Sr2MgMoO6-δ as perspective anode for solid oxide fuel cells. J. Solid State Electrochem. 2018, 22, 1209–1215. [Google Scholar] [CrossRef]
- Skutina, L.; Filonova, E.; Medvedev, D.; Maignan, A. Undoped Sr2MMoO6-δ, double perovskite molybdates (M = Ni, Mg, Fe) as promising anode materials for solid oxide fuel cells. Materials 2021, 14, 1715. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Xing, Y.; Xie, Y.; Shi, N.; Xu, J.; Xia, C. Vanadium-doped strontium molybdate with exsolved Ni Nanoparticles as anode material for solid oxide fuel cells, ACS Appl. Mater. Interf. 2019, 11, 42271–42279. [Google Scholar] [CrossRef] [PubMed]
- Osinkin, D.A.; Beresnev, S.M.; Khodimchuk, A.V.; Korzun, I.V.; Lobachevskaya, N.I.; Suntsov, A.Y. Functional properties and electrochemical performance of Ca-doped Sr2-xCaxFe1.5Mo0.5O6-δ as anode for solid oxide fuel cells. J. Solid State Electrochem. 2019, 23, 627–634. [Google Scholar] [CrossRef]
- Istomin, S.Y.; Kotova, A.I.; Lyskov, N.V.; Mazo, G.N.; Antipov, E.V. Pr5Mo3O16 + δ: A new anode material for solid oxide fuel cells. Russ. J. Inorg. Chem. 2018, 63, 1291–1296. [Google Scholar] [CrossRef]
- Zhang, J.; Lei, L.; Li, H.; Chen, F.; Han, M. A practical approach for identifying various polarization behaviors of redox-stable electrodes in symmetrical solid oxide fuel cells. Electrochim. Acta 2021, 384, 138340. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Yang, Z.; Lei, Z.; Jin, C.; Liu, Y.; Wang, Y.; Peng, S. Co-substituted Sr2Fe1.5Mo0.5O6-δ as anode materials for solid oxide fuel cells: Achieving high performance via nanoparticle exsolution. J. Power Sources 2019, 438, 226989. [Google Scholar] [CrossRef]
- Li, Y.; Zou, S.; Ju, J.; Xia, C. Characteristics of nano-structured SFM infiltrated onto YSZ backbone for symmetrical and reversible solid oxide cells. Solid State Ion. 2018, 319, 98–104. [Google Scholar] [CrossRef]
- Huang, B.; Wang, S.R.; Liu, R.Z.; Ye, X.F.; Nie, H.W.; Sun, X.F.; Wen, T.L. Performance of La0.75Sr0.25Cr0.5Mn0.5O3−δ perovskite-structure anode material at lanthanum gallate electrolyte for IT-SOFC running on ethanol fuel. J. Power Sources 2007, 167, 39–46. [Google Scholar] [CrossRef]
- Bastidas, D.M.; Tao, S.; Irvine, J.T.S. A symmetrical solid oxide fuel cell demonstrating redox stable perovskite electrodes. J. Mater. Chem. 2006, 16, 1603–1605. [Google Scholar] [CrossRef]
- Ruiz-Morales, J.C.; Canales-Vázquez, J.; Peña-Martínez, J.; López, D.M.; Núñez, P. On the simultaneous use of La0.75Sr0.25Cr0.5Mn0.5O3−δ as both anode and cathode material with improved microstructure in solid oxide fuel cells. Electrochim. Acta 2006, 52, 278–284. [Google Scholar] [CrossRef]
- Mohammadi, A.; Wu, T.; Smirnova, A.L.; Pusz, J.; Sammes, N.M. All-perovskite solid oxide fuel cells, synthesis and characterization. J. Fuel Cell Sci. Technol. 2009, 6, 21308. [Google Scholar] [CrossRef]
- Lay, E.; Gauthier, G.; Dessemond, L. Preliminary studies of the new Ce-doped La / Sr chromo-manganite series as potential SOFC anode or SOEC cathode materials. Solid State Ion. 2011, 189, 91–99. [Google Scholar] [CrossRef]
- Fu, Q.X.; Tietz, F.; Lersch, P.; Stöver, D. Evaluation of Sr- and Mn-substituted LaAlO3 as potential SOFC anode materials. Solid State Ion. 2006, 177, 1059–1069. [Google Scholar] [CrossRef]
- Sengodan, S.; Yeo, H.J.; Shin, J.Y.; Kim, G. Intermediate-temperature solid oxide fuel cells using hydrocarbon fuels. J. Power Sources 2011, 196, 3083–3088. [Google Scholar] [CrossRef]
- Sengodan, S.; Choi, S.; Jun, A.; Shin, T.H.; Ju, Y.-W.; Jeong, H.Y.; Shin, J.Y.; Irvine, J.T.S.; Kim, G. Layered oxygen-deficient double perovskite as an efficient and stable anode for direct hydrocarbon solid oxide fuel cells. Nat. Mater. 2015, 14, 205–209. [Google Scholar] [CrossRef]
- Tomkiewicz, A.C.; Tamimi, M.A.; Huq, A.; McIntosh, S. Structural analysis of PrBaMn2O5+δ under SOFC anode conditions by in-situ neutron powder diffraction. J. Power Sources 2016, 330, 240–245. [Google Scholar] [CrossRef] [Green Version]
- Felli, A.; Trovarelli, A.; Boaro, M. Investigation of the redox behavior of double perovskite PrBaMn2O5+δ. ECS Trans. 2021, 103, 1479–1489. [Google Scholar] [CrossRef]
- Chen, M.; Xu, X.; Bao, S.; Ren, G.-K.; Lin, Y.-H.; Jacobson, A.J.; Ma, J.; Nan, C.-W.; Chen, C. Remarkable switching of transport properties and surface exchange kinetics in epitaxial PrBaMn2O5+δ films. Acta Mater. 2020, 186, 517–522. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, Y.; Zheng, Y.; Chen, H.; Ge, L.; Guo, L. PrBaMn2O5+δ with praseodymium oxide nano-catalyst as electrode for symmetrical solid oxide fuel cells. Appl. Catal. B Environ. 2019, 257, 117868. [Google Scholar] [CrossRef]
- Sun, Y.-F.; Zhang, Y.-Q.; Hua, B.; Behnamian, Y.; Li, J.; Cui, S.-H.; Li, J.H.; Luo, J.-L. Molybdenum doped Pr0.5Ba0.5MnO3-δ (Mo-PBMO) double perovskite as a potential solid oxide fuel cell anode material. J. Power Sources 2016, 301, 237–241. [Google Scholar] [CrossRef]
- Choi, S.; Sengodan, S.; Park, S.; Ju, Y.-W.; Kim, J.; Hyodo, J.; Jeong, H.Y.; Ishihara, T.; Shin, J.; Kim, G. A robust symmetrical electrode with layered perovskite structure for direct hydrocarbon solid oxide fuel cells: PrBa0.8Ca0.2Mn2O5+δ. J. Mater. Chem. 2016, 4, 1747–1753. [Google Scholar] [CrossRef]
- Kwon, Y.; Kang, S.; Bae, J. Development of a PrBaMn2O5+δ-La0.8Sr0.2Ga0.85Mg0.15O3-δ composite electrode by scaffold infiltration for reversible solid oxide fuel cell applications. Int. J. Hydrogen Energy 2020, 45, 1748–1758. [Google Scholar] [CrossRef]
- Zhang, B.; Wan, Y.; Hua, Z.; Tang, K.; Xia, C. Tungsten-doped PrBaFe2O5+δ double perovskite as a high-performance electrode material for symmetrical solid oxide fuel cells. ACS Appl. Energy Mater. 2021, 4, 8401–8409. [Google Scholar] [CrossRef]
- Managutti, P.B.; Tymen, S.; Liu, X.; Hernandez, O.; Prestipino, C.; Le Gal La Salle, A.; Paul, S.; Jalowiecki-Duhamel, L.; Dorcet, V.; Billard, A.; et al. Exsolution of Ni nanoparticles from A-Site-deficient layered double perovskites for dry reforming of methane and as an anode material for a solid oxide fuel cell. ACS Appl. Mater. Interf. 2021, 13, 35719–35728. [Google Scholar] [CrossRef]
- Vecino-Mantilla, S.; Simon, P.; Huvé, M.; Gauthier, G.; Gauthier-Maradei, P. Methane steam reforming in water-deficient conditions on a new Ni-exsolved Ruddlesden-Popper manganite: Coke formation and H2S poisoning. Int. J. Hydrogen Energy 2020, 45, 27145–27159. [Google Scholar] [CrossRef]
- Lo Faro, M.; La Rosa, D.; Nicotera, I.; Antonucci, V.; Aricò, A.S. Electrochemical behaviour of propane-fed solid oxide fuel cells based on low Ni content anode catalysts. Electrochim. Acta 2009, 54, 5280–5285. [Google Scholar] [CrossRef]
- Martinez-Arias, A.; Hungría, A.B.; Fernandez-Garcia, M.; Iglesias-Juez, A.; Conesa, J.C.; Mather, G.C.; Munuera, G. Cerium–terbium mixed oxides as potential materials for anodes in solid oxide fuel cells. J. Power Sources 2005, 51, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Marina, O.A.; Bagger, C.; Primdahl, S.; Mogensen, M.A. Solid oxide fuel cell with a gadolinia-doped ceria anode: Preparation and performance. Solid State Ion. 1999, 123, 199–208. [Google Scholar] [CrossRef]
- Ramirez-Cabrera, E.; Atkinson, A.; Chadwick, D. Catalytic steam reforming of methane over Ce0.9Gd0.1O2−x. Appl. Catal. B Environ. 2004, 47, 127–131. [Google Scholar] [CrossRef]
- Ramirez-Cabrera, E.; Laosiripojana, N.; Atkinson, A.; Chadwick, D. Methane conversion over Nb-doped ceria. Catal. Today 2003, 78, 433–438. [Google Scholar] [CrossRef]
- Cai, G.; Liu, R.; Zhao, C. Anode performance of Mn-doped ceria—ScSZ for solid oxide fuel cell. J. Solid State Electrochem. 2011, 15, 147–152. [Google Scholar] [CrossRef]
- Song, S.; Fuentes, R.O.; Baker, R.T. Nanoparticulate ceria—Zirconia anode materials for intermediate temperature solid oxide fuel cells using hydrocarbon fuels. J. Mater. Chem. 2010, 20, 9760–9769. [Google Scholar] [CrossRef]
- Larrondo, S.; Vidal, M.B.; Irigoyen, A.; Craievich, D.; Lamas, I.; Fábregas, G.; Lascalea, N.; Walsöe de Reca, N. Amadeo, Preparation and characterization of Ce/Zr mixed oxides and their use as catalysts for the direct oxidation of dry CH4. Catal. Today. 2005, 107, 53–59. [Google Scholar] [CrossRef]
- Sabolsky, E.M.; Seabaugh, M.; Sabolsky, K.; Ibanez, S.A.; Zhong, Z. SOFC cells and stacks for complex fuels. ECS Trans. 2007, 7, 503–510. [Google Scholar] [CrossRef]
- Jin, C.; Yang, C.; Zhao, F.; Cof, A.; Chen, F. Direct-methane solid oxide fuel cells with Cu1.3Mn1.7O4 spinel internal reforming layer. Electrochem. Commun. 2010, 12, 1450–1452. [Google Scholar] [CrossRef]
- Boulfrad, S.; Cassidy, M.; Irvine, J.T.S. NbTi0.5Ni0.5O4 as anode compound material for SOFCs. Solid State Ion. 2011, 197, 37–41. [Google Scholar] [CrossRef]
- Li, Q.; Thangadurai, V. Novel Nd2WO6 -type Sm2−xAxM1−yByO6−δ (A = Ca, Sr; M = Mo, W; B = Ce, S. Ni ) mixed conductors. J. Power Sources 2011, 196, 169–178. [Google Scholar] [CrossRef]
- Shin, T.H.; Ida, S.; Ishihara, T. Doped CeO2-LaFeO3 composite oxide as an active anode for direct hydrocarbon-type solid oxide fuel cells. J. Am. Chem. Soc. 2011, 3, 19399–19407. [Google Scholar] [CrossRef]
- Runge, H.; Greenblatt, M. Structure and conductivity investigations of alkaline earth substituted uranium oxide, U1-XMxO2±δ (M = Mg, Ca, Sr) for solid oxide fuel cell applications. Solid State Ion. 2006, 177, 269–274. [Google Scholar] [CrossRef]
- Kim, G.; Lee, S.; Shin, J.Y.; Corre, G.; Irvine, J.T.S.; Vohs, J.M.; Gorte, R.J. Investigation of the structural and catalytic requirements for high-performance SOFC anodes formed by infiltration of LSCM. Electrochem. Solid-State Lett. 2009, 12, 48–52. [Google Scholar] [CrossRef] [Green Version]
- Aruna, S.T.; Muthuraman, M.; Patil, K.C. Synthesis and properties of Ni-YSZ cermet: Anode material for solid oxide fuel cells. Solid State Ion. 1998, 111, 45–51. [Google Scholar] [CrossRef]
- Itoh, H.; Yamamoto, T.; Mori, M.; Abe, T. Sintering behaviour and performance of anode materials for SOFC. In Proceedings of the Fourth International Symposium on Solid Oxide Fuel Cells (SOFC’s IV), Yokohama, Japan, 18–23 June 1995; Dokiya, M., Yamamoto, O., Tagawa, H., Singhal, S.C., Eds.; Electrochemical Society: Pennington, NJ, USA, 1995; pp. 639–648. [Google Scholar]
- Steele, B.C.H. State-of-the-art SOFC ceramic materials. In Proceedings of the 1st European Solid Oxide Fuel Cell Forum, Lucerne, Switzerland, 3–7 October 1994; pp. 375–397. [Google Scholar]
- Prakash, B.S.; Kumar, S.S.; Aruna, S.T. Properties and development of Ni/YSZ as an anode material in solid oxide fuel cell: A review. Renew. Sustain. Energy Rev. 2014, 36, 149–179. [Google Scholar] [CrossRef]
- Isaacs, H.S.; Olmer, L.J.; Schouler, E.J.L.; Yang, C.Y. Electrode reactions at solid oxide electrolytes. Solid State Ion. 1981, 3, 503–507. [Google Scholar] [CrossRef]
- Burch, R.; Hayes, M.J. C-H bond activation in hydrocarbon oxidation on solid catalysts, J. Mol. Catal. A Chem. 1995, 100, 13–33. [Google Scholar] [CrossRef]
- Koh, J.H.; Yoo, Y.S.; Park, J.W.; Lim, H.C. Carbon deposition and cell performance of Ni-YSZ anode support SOFC with methane fuel. Solid State Ion. 2002, 149, 157–166. [Google Scholar] [CrossRef]
- Eguchi, K.; Kojo, H.; Takeguchi, T.; Kikuchi, R.; Sasaki, K. Fuel flexibility in power generation by solid oxide fuel cells. Solid State Ion. 2002, 152, 411–416. [Google Scholar] [CrossRef]
- Wang, D.; Wong, S.I.; Sunarso, J.; Xu, M.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z.A. Direct n-butane solid oxide fuel cell using Ba(Zr0.1Ce0.7Y0.1Yb0.1)0.9Ni0.05Ru0.05 O3−δ perovskite as the reforming layer. ACS Appl Mater. Interf. 2021, 13, 20105–20113. [Google Scholar] [CrossRef]
- Sumi, H.; Shimada, H.; Yamaguchi, T.; Hamamoto, K.; Suzuki, T.; Fujishiro, Y. Development of microtubular solid oxide fuel cells using hydrocarbon fuels. In Advances in Solid Oxide Fuel Cells and Electronic Ceramics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 93–104. [Google Scholar]
- Zha, S.; Moore, A.; Abernathy, H.; Liu, M. GDC-Based Low-Temperature SOFCs Powered by Hydrocarbon Fuels. J. Electrochem. Soc. 2004, 151, A1128–A1133. [Google Scholar] [CrossRef]
- Muccillo, R.; Muccillo, E.N.S.; Fonseca, F.C.; de Florio, D.Z. Characteristics and performance of electrolyte-supported solid oxide fuel cells under ethanol and hydrogen. J. Electrochem. Soc. 2008, 155, B232–B235. [Google Scholar] [CrossRef]
- Campbell, C.T.; Peden, C.H.F. Oxygen vacancies and catalysis on ceria surfaces. Science 2005, 309, 713–714. [Google Scholar] [CrossRef]
- Qiu, P.; Yang, X.; Sun, S.; Jia, L.; Li, J.; Chen, F. Enhanced electrochemical performance and durability for direct CH4–CO2 solid oxide fuel cells with an on-cell reforming layer. Int. J. Hydrogen Energy 2021, 46, 22974–22982. [Google Scholar] [CrossRef]
- Yano, M.; Kawai, T.; Okamoto, K.; Nagao, M.; Sano, M.; Tomita, A.; Hibino, T. Single-chamber SOFCs using dimethyl ether and ethanol. J. Electrochem. Soc. 2007, 154, B865–B870. [Google Scholar] [CrossRef]
- Park, S.; Vohs, J.M.; Gorte, R.J. Direct oxidation of hydrocarbons in a solid-oxide fuel cell. Nature 2000, 404, 265–267. [Google Scholar] [CrossRef] [PubMed]
- Skarmoutsos, D.; Nikolopoulos, P.; Tietz, F.; Vinke, I.C. Physical characterization of Y0.25Zr0.60Ti0.15O2-x and its performance as a Ni/Y0.25Zr0.60Ti0.15O2−x anode cermet in an SOFC. Solid State Ion. 2004, 170, 153–158. [Google Scholar] [CrossRef]
- Gorte, R.J.; Kim, H.; Vohs, J.M. Novel SOFC anodes for the direct electrochemical oxidation of hydrocarbon. J. Power Sources 2002, 106, 10–15. [Google Scholar] [CrossRef] [Green Version]
- Kaklidis, N.; Pekridis, G.; Besikiotis, V.; Athanasiou, C.; Marnellos, G.E. Direct electro-oxidation of acetic acid in a solid oxide fuel cell. Solid State Ion. 2012, 225, 398–407. [Google Scholar] [CrossRef]
- Ye, X.; Wang, S.R.; Hu, Q.; Chen, J.Y.; Wen, T.L.; Wen, Z.Y. Improvement of Cu—CeO2 anodes for SOFCs running on ethanol fuels. Solid State Ion. 2009, 180, 276–281. [Google Scholar] [CrossRef]
- Ramírez-Cabrera, E.; Atkinson, A.; Chadwick, D. The influence of point defects on the resistance of ceria to carbon deposition in hydrocarbon catalysis. Solid State Ion. 2000, 136, 825–831. [Google Scholar] [CrossRef]
- Lu, X.C.; Zhu, J.H. Cu(Pd)-impregnated La0.75Sr0.25Cr0.5Mn0.5O3 − δ anodes for direct utilization of methane in SOFC. Solid State Ion. 2007, 178, 1467–1475. [Google Scholar] [CrossRef]
- Akdeniz, Y.; Timurkutluk, B.; Timurkutluk, C. Development of anodes for direct oxidation of methane fuel in solid oxide fuel cells. Int. J. Hydrogen Energy 2016, 41, 10021–10029. [Google Scholar] [CrossRef]
- Ringuedé, A.; Bronine, D.; Frade, J.R. Ni1−xCox/YSZ cermet anodes for solid oxide fuel cells. Electrochim. Acta 2002, 48, 437–442. [Google Scholar] [CrossRef]
- Kurokawa, H.; Yang, L.; Jacobson, C.P.; De Jonghe, L.C.; Visco, S.J. Y-doped SrTiO3 based sulfur tolerant anode for solid oxide fuel cells. J. Power Sources 2007, 164, 510–518. [Google Scholar] [CrossRef]
- Morimoto, K.; Shimotsu, M. The Fuel Electrode Material Using Fe-YSZ Cermet. ECS Proc. 1995, 1, 769–780. [Google Scholar] [CrossRef]
- Bernardo, C.A.; Alstrup, I.; Rostrup-Nielsen, J.R. Carbon deposition and methane steam reforming on silica-supported Ni-Cu catalysts. J. Catal. 1985, 96, 517–534. [Google Scholar] [CrossRef]
- Kasyanova, A.V.; Tarutina, L.R.; Rudenko, A.O.; Lyagaeva, J.G.; Medvedev, D.A. Ba(Ce,Zr)O3-based electrodes for protonic ceramic electrochemical cells: Towards highly compatible functionality and triple-conducting behavior. Russ. Chem. Rev. 2020, 89, 667–692. [Google Scholar] [CrossRef]
- Bae, K.; Kim, D.H.; Choi, H.J.; Son, J.-W.; Shim, J.H. High-Performance Protonic Ceramic Fuel Cells with 1 µm Thick Y:Ba(Ce, Zr)O3 Electrolytes. Advan. Energy Mater. 2018, 8, 1801315. [Google Scholar] [CrossRef]
- Onishi, T.; Han, D.; Noda, Y.; Hatada, N.; Majima, M.; Uda, T. Evaluation of performance and durability of Ni-BZY cermet electrodes with BZY electrolyte. Solid State Ion. 2018, 317, 127–135. [Google Scholar] [CrossRef]
- Pers, P.; Mao, V.; Taillades, M.; Taillades, G. Electrochemical behavior and performances of Ni-BaZr0·1Ce0·7Y0.1Yb0.1O3−δ cermet anodes for protonic ceramic fuel cell. Int. J. Hydrogen Energy. 2018, 43, 2402–2409. [Google Scholar] [CrossRef]
- Nasani, N.; Ramasamy, D.; Antunes, I.; Perez, J.; Fagg, D.P. Electrochemical behaviour of Ni-BZO and Ni-BZY cermet anodes for Protonic Ceramic Fuel Cells (PCFCs)—A comparative study. Electrochim. Acta 2015, 154, 7–13. [Google Scholar] [CrossRef]
- Nasani, N.; Ramasamy, D.; Brandão, A.D.; Yaremchenko, A.A.; Fagg, D.P. The impact of porosity, pH2 and pH2O on the polarisation resistance of Ni-BaZr0.85Y0.15O3-δ cermet anodes for Protonic Ceramic Fuel Cells (PCFCs). Int. J. Hydrogen Energy 2014, 39, 21231–21241. [Google Scholar] [CrossRef]
- Li, M.; Hua, B.; Luo, J.-L.; Jiang, S.P.; Pu, J.; Chi, B.; Jian, L. Carbon-tolerant Ni-based cermet anodes modified by proton conducting yttrium- and ytterbium-doped barium cerates for direct methane solid oxide fuel cells. J. Mater. Chem. A 2015, 3, 21609–21617. [Google Scholar] [CrossRef]
- Hong, K.; Sutanto, S.N.; Lee, J.A.; Hong, J. Ni-based bimetallic nano-catalysts anchored on BaZr0.4Ce0.4Y0.1Yb0.1O3−δ for internal steam reforming of methane in a low-temperature proton-conducting ceramic fuel cell. J. Mater. Chem. A. 2021, 9, 6139–6151. [Google Scholar] [CrossRef]
- Nishikawa, R.; Nishino, H.; Brito, M.E.; Kakinuma, K. Synthesis and evaluation of double-layer electrodes using a Ni-BaCe0.50Zr0.27Y0.20Ni0.03O3-δ cermet with a fused-aggregate network structure as the hydrogen electrode of solid oxide cells. J. Ceram. Soc. Jpn. 2018, 126, 208–213. [Google Scholar] [CrossRef] [Green Version]
- Stange, M.; Stefan, E.; Denonville, C.; Larring, Y.; Rørvik, P.M.; Haugsrud, R. Development of novel metal-supported proton ceramic electrolyser cell with thin film BZY15–Ni electrode and BZY15 electrolyte. Int. J. Hydrogen Energy 2017, 42, 13454–13462. [Google Scholar] [CrossRef]
- Miyazaki, K.; Okanishi, T.; Muroyama, H.; Matsui, T.; Eguchi, K. Development of Ni–Ba(Zr,Y)O3 cermet anodes for direct ammonia-fueled solid oxide fuel cells. J. Power Sources 2017, 365, 148–154. [Google Scholar] [CrossRef]
- Gorte, R.J.; Park, S.; Vohs, J.M.; Wang, C. Anodes for direct oxidation of dry hydrocarbons in a solid oxide fuel cell. Adv. Mater. 2000, 12, 1465–1469. [Google Scholar] [CrossRef]
- Park, S.; Craciun, R.; Vohs, J.M.; Gorte, R.J. Direct oxidation of hydrocarbons in a solid oxide fuel cell: I. Methane oxidation. J. Electrochem. Soc. 1999, 146, 3603–3605. [Google Scholar] [CrossRef]
- Sinfelt, J.H.; Carter, J.L.; Yates, D.J.C. Catalytic hydrogenolysis and dehydrogenation over copper-nickel alloys. J. Catal. 1972, 24, 283–296. [Google Scholar] [CrossRef]
- Rodriguez, N.M.; Kim, M.S.; Baker, R.T.K. Deactivation of copper nickel-catalysts due to changes in surface composition. J. Catal. 1993, 140, 16–29. [Google Scholar] [CrossRef]
- Avdeeva, L.B.; Goncharova, O.V.; Kochubey, D.I.; Zaikovskii, V.I.; Plyasova, L.M.; Novgorodov, B.N.; Shaikhutdinov, S.K. Coprecipitated Ni-alumina and Ni-Cu-alumina catalysts of methane decomposition and carbon deposition. II. Evolution of the catalysts in reaction. Appl. Catal. A Gen. 1996, 141, 117–129. [Google Scholar] [CrossRef]
- Lu, Z. Study on new copper-containing SOFC anode materials. J. Alloys Compd. 2002, 334, 299–303. [Google Scholar] [CrossRef]
- Kim, H.; Lu, C.; Worrell, W.L.; Vohs, J.M.; Gorte, R.J. Cu-Ni Cermet anodes for direct oxidation of methane in solid-oxide fuel cells. J. Electrochem. Soc. 2002, 149, A247–A250. [Google Scholar] [CrossRef]
- Woo, E.; Moon, H.; Park, M.; Hoon, S. Fabrication and characterization of Cu—Ni—YSZ SOFC anodes for direct use of methane via Cu-electroplating. Int. J. Hydrogen Energy 2009, 34, 5537–5545. [Google Scholar]
- Zhao, C.H.; Liu, R.Z.; Shao, L.; Wang, S.R.; Wen, T.L. Electrochemistry communications effects of Cuo addition to anode on the electrochemical performances of cathode-supported solid oxide fuel cells. Electrochem. Commun. 2009, 11, 2300–2303. [Google Scholar] [CrossRef]
- Islam, S.; Hill, J.M. Preparation of Cu—Ni/YSZ solid oxide fuel cell anodes using microwave irradiation. J. Power Sources 2011, 196, 5091–5094. [Google Scholar] [CrossRef]
- Senthil Kumar, S.; Jayaram, V.; Aruna, S.T. Co-fired anode-supported solid oxide fuel cell for internal reforming of hydrocarbon fuel. Energy Ecol. Environ. 2021, 6, 55–68. [Google Scholar] [CrossRef]
- Fu, C.J.; Chan, S.H.; Ge, X.M.; Liu, Q.L.; Pasciak, G. A promising Ni-Fe bimetallic anode for intermediate-temperature SOFC based on Gd-doped ceria electrolyte. Int. J. Hydrogen Energy 2011, 6, 2–9. [Google Scholar] [CrossRef]
- Kan, H.; Lee, H. Enhanced stability of Ni—Fe/GDC solid oxide fuel cell anodes for dry methane fuel. Catal. Commun. 2010, 12, 36–39. [Google Scholar] [CrossRef]
- Da Paz Fiuza, R.; Da Silva, M.A.; Boaventura, J.S. Development of Fe-Ni/YSZ-GDC electro-catalysts for application as SOFC Anodes: XRD and TPR characterization, and evaluation in ethanol steam reforming reaction. In Proceedings of the 18th World Hydrogen Energy Conference, Essen, Germany, 16–21 May 2010; pp. 273–280. [Google Scholar]
- Lu, X.C.; Zhu, J.H.; Bi, Z.H. Fe alloying effect on the performance of the Ni anode in hydrogen fuel. Solid State Ion. 2009, 180, 265–270. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, J.S.; Han, J.; Seo, J.; Lee, C.; Hong, S. Surface alloying of a Co film on the Cu(001) surface. Surf. Sci. 2000, 453, 47–58. [Google Scholar] [CrossRef]
- Gross, M.D.; Vohs, J.M.; Gorte, R.J. A study of thermal stability and methane tolerance of Cu-based SOFC anodes with electrodeposited Co. Electrochim. Acta 2007, 52, 1951–1957. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-I.; Ahn, K.; Vohs, J.M.; Gorte, R.J. Cu-Co bimetallic anodes for direct utilization of methane in SOFCs. Electrochem. Solid-State Lett. 2005, 8, A48. [Google Scholar] [CrossRef]
- Sarruf, B.; Hong, J.-E.; Robert, S.-W.; Miranda, P. CeO2-Co3O4-CuO anode for direct utilisation of methane or ethanol in solid oxide fuel cells. Int. J. Hydrogen Energy 2018, 43, 6340–6351. [Google Scholar] [CrossRef]
- Ishihara, T.; Yan, J.; Shinagawa, M.; Matsumoto, H. Ni—Fe bimetallic anode as an active anode for intermediate temperature SOFC using LaGaO3 based electrolyte film. Electrochim. Acta 2006, 52, 1645–1650. [Google Scholar] [CrossRef]
- Grgicak, C.M.; Pakulska, M.M.; O’Brien, J.S.; Giorgi, J.B. Synergistic effects of Ni1-xCox-YSZ and Ni1-xCux-YSZ alloyed cermet SOFC anodes for oxidation of hydrogen and methane fuels containing H2S. J. Power Sources 2008, 183, 26–33. [Google Scholar] [CrossRef]
- Brien, J.S.O.; Giorgi, J.B. Solid oxide fuel cell with NiCo—YSZ cermet anode for oxidation of CO / H2 fuel mixtues. J. Power Sources 2012, 200, 14–20. [Google Scholar]
- Kaur, G.; Basu, S. Performance studies of copper-iron/ceria-yttria stabilized zirconia anode for electro-oxidation of butane in solid oxide fuel cells. J. Power Sources 2013, 241, 783–790. [Google Scholar] [CrossRef]
- Hua, B.; Li, M.; Zhang, Y.-Q.; Chen, J.; Sun, Y.-F.; Yan, N.; Li, J.; Luo, J.-L. Facile Synthesis of highly active and robust Ni–Mo bimetallic electrocatalyst for hydrocarbon oxidation in solid oxide fuel cells. ACS Energy Lett. 2016, 1, 225–230. [Google Scholar] [CrossRef]
- Ma, Y.; Guan, G.; Hao, X.; Zuo, Z.; Huang, W.; Phanthong, P.; Kusakabe, K.; Abudula, A. Highly-efficient steam reforming of methanol over copper modified molybdenum carbide. RSC Adv. 2014, 4, 44175–44184. [Google Scholar] [CrossRef]
- Hanping, D.; Desheng, Z.; Shun, L.; Wei, W.; Yating, Y.; Yingchao, Y.; Zetian, T. Electricity generation in dry methane by a durable ceramic fuel cell with high-performing and coking resistant layered perovskite anode. Appl. Energy. 2019, 233–234, 37–43. [Google Scholar]
- Qi, Y.; Fengtao, C.; Chao, M.; Chunwen, S.; Siqi, S.; Liquan, C. Enhanced coking tolerance of MgO-modified Ni Cermet Anode for Hydrocarbon Fueled Solid Oxide Fuel Cells. J. Mat. Chem. A 2016, 4, 18031–18036. [Google Scholar]
- Mingfei, L.; Ranran, P.; Dehua, D.; Jianfeng, G.; Xingqin, L.; Guangyao, M. Direct liquid methanol-fueled solid oxide fuel cell. J. Power Sources 2008, 185, 188–192. [Google Scholar]
- Lilu, L.; Qi, Y.; Wei, Y.; Xingguo, Q.; Chunwen, S.; Liquan, C. Li/Na Modified Ni-SDC Anode for Methane-fueled Solid Oxide Fuel Cells. ECS Trans. 2015, 68, 1403–1409. [Google Scholar]
- Byeong, W.K.; Caleb, E.; Joe, B.; Jinsoo, K.; Grant, M.N.; Su, H. Molybdenum dioxide-based anode for solid oxide fuel cell applications. J. Power Sources 2013, 243, 203–210. [Google Scholar]
- Jae-ha, M.; Sun-Dong, K.; Tae Ho, S.; Daehee, L.; John, T.S.I.; Jooho, M.; Sang-Hoon, H. Nano-composite structural Ni–Sn alloy anodes for high performance and durability of direct methane fueled SOFCs. J. Mat. Chem. A 2015, 3, 13801–13806. [Google Scholar]
- Byeong, W.K.; Shuozhen, H.; Qian, H.; Oscar, G.M.-F.; Chang, H.O.; Sung, P.Y.; Jinsoo, K.; Joe, B.; Louis, S.; Grant, M.N.; et al. Nickel-based anode with microstructured molybdenum dioxide internal reformer for liquid hydrocarbon-fueled solid oxide fuel cells. Appl. Cat. B Environ. 2015, 179, 439–444. [Google Scholar]
- Zhong, H.; Ishihara, T.; Matsumoto, H. Ni-Fe-LaGaO3 based Alloy Anode Cermet for Direct Hydrocarbon type Solid Oxide Fuel Cell using LaGaO3 Electrolyte. Mat. Sci. Forum 2010, 638–642, 1112–1117. [Google Scholar] [CrossRef]
- Gore, C.M.; Lee, K.T.; Yoon, H.S.; Wachsman, E.D. Porous GDC scaffold anodes for lower temperature, hydrocarbon-fueled solid oxide fuel cells. ECS Trans. 2013, 50, 53–62. [Google Scholar] [CrossRef]
- Kishimoto, H.; Horita, T.; Yamaji, K.; Brito, M.E.; Xiong, Y.-P.; Yokokawa, H. Sulfur poisoning on SOFC Ni anodes: Thermodynamic analyses within local equilibrium anode reaction model. J. Electrochem. Soc. 2010, 157, B802–B813. [Google Scholar] [CrossRef]
- Lin, R.Y.; Hu, D.C.; Chang, Y.A. Thermodynamics and phase relationships of transition metal-sulfur systems: II. The nickel-sulfur system. Metall. Mater. Trans. B 1978, 9, 531–538. [Google Scholar] [CrossRef]
- Ishikura, A.; Sakuno, S.; Komiyama, N.; Sasatsu, H.; Masuyama, N.; Itoh, H.; Yasumoto, K. Influence of H2S poisoning on anode layer of SOFC. ECS Trans. 2007, 7, 845–850. [Google Scholar] [CrossRef]
- Matsuzaki, Y.; Yasuda, I. The poisoning effect of sulfur-containing impurity gas on a SOFC anode: Part, I. Dependence on temperature, time, and impurity concentration. Solid State Ion. 2000, 132, 261–269. [Google Scholar] [CrossRef]
- Gong, M.; Liu, X.; Trembly, J.; Johnson, C. Sulfur-tolerant anode materials for solid oxide fuel cell application. J. Power Sources 2007, 168, 289–298. [Google Scholar] [CrossRef]
- Trembly, J.P.; Marquez, A.I.; Ohrn, T.R.; Bayless, D.J. Effects of coal syngas and H2S on the performance of solid oxide fuel cells: Single-cell tests. J. Power Sources 2006, 158, 263–273. [Google Scholar] [CrossRef]
- Marquez, A.I.; Ohrn, T.R.; Trembly, J.P.; Ingram, D.C.; Bayless, D.J. Effects of coal syngas and H2S on the performance of solid oxide fuel cells. Part 2. Stack tests. J. Power Sources 2007, 164, 659–667. [Google Scholar] [CrossRef]
- Dong, J.; Cheng, Z.; Zha, S.; Liu, M. Identification of nickel sulfides on Ni–YSZ cermet exposed to H2 fuel containing H2S using Raman spectroscopy. J. Power Sources 2006, 156, 461–465. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Mechanisms of catalyst deactivation. Appl. Catal. A Gen. 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Sasaki, K.; Susuki, K.; Iyoshi, A.; Uchimura, M.; Imamura, N.; Kusaba, H.; Teraoka, Y.; Fuchino, H. H2S poisoning of solid oxide fuel cells. J. Electrochem. Soc. 2006, 153, A2023–A2029. [Google Scholar] [CrossRef]
- Aguilar, L.; Zha, S.; Cheng, Z.; Winnick, J.; Liu, M. A solid oxide fuel cell operating on hydrogen sulfide (H2S) and sulfur-containing fuels. J. Power Sources 2004, 135, 17–24. [Google Scholar] [CrossRef]
- Aguilar, L.; Zha, S.; Li, S.; Winnick, J.; Liu, M. Sulfur-tolerant materials for the hydrogen sulfide SOFC. ECS Solid State Lett 2004, 2, 30332. [Google Scholar] [CrossRef]
- Aguilar, L. Sulfur Tolerant Materials for the Hydrogen Sulfide Solid Oxide Fuel Cell. Ph.D. Thesis, Georgia Institute of Technology, Atlanta, GA, USA, December 2004. [Google Scholar]
- Danilovic, N.; Luo, J.; Chuang, K.T.; Sanger, A.R. Effect of substitution with Cr3+ and addition of Ni on the physical and electrochemical properties of Ce0.9Sr0.1VO3 as a H2S-active anode for solid oxide fuel cells. J. Power Sources 2009, 194, 252–262. [Google Scholar] [CrossRef]
- Mukundan, R.; Brosha, E.L.; Garzon, F.H. Sulfur tolerant anodes for SOFCs. Electrochem. Solid-State Lett. 2004, 7, A5–A7. [Google Scholar] [CrossRef]
- Cheng, J.; Gong, J.; Yue, S.; Jiang, Y.; Hou, X.; Ma, J.; Yao, Y.; Jiang, C. Electrochemical investigation of La0.4Sr0.6TiO3 synthesized in air for SOFC application. J. Appl. Electrochem. 2021, 51, 1175–1188. [Google Scholar] [CrossRef]
- Shatynski, S.R. The thermochemistry of transition metal sulfides. Oxid. Met. 1977, 11, 307–320. [Google Scholar] [CrossRef]
- Zha, S.; Cheng, Z.; Liu, M. Sulfur poisoning and regeneration of Ni-based anodes in solid oxide fuel cells. J. Electrochem. Soc. 2007, 154, B201–B206. [Google Scholar] [CrossRef]
- Lussier, A.; Sofie, S.; Dvorak, J.; Idzerda, Y.U. Mechanism for SOFC anode degradation from hydrogen sulfide exposure. Int. J. Hydrogen Energy 2008, 33, 3945–3951. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, S.P.; He, H.Q.; Chen, X.; Ma, J.; Song, X.C. A comparative study of H2S poisoning on electrode behavior of Ni/YSZ and Ni/GDC anodes of solid oxide fuel cells. Int. J. Hydrogen Energy 2010, 35, 12359–12368. [Google Scholar] [CrossRef]
- Brightman, E.; Ivey, D.G.; Brett, D.J.L.; Brandon, N.P. The effect of current density on H2S-poisoning of nickel-based solid oxide fuel cell anodes. J. Power Sources 2011, 196, 7182–7187. [Google Scholar] [CrossRef]
- Li, M.; Hua, B.; Luo, J.-L.; Jiang, S.P.; Pu, J.; Chi, B.; Li, J. Enhancing sulfur tolerance of Ni-based cermet anodes of solid oxide fuel cells by ytterbium-doped barium cerate infiltration. ACS Appl. Mater. Interfaces 2016, 8, 10293–10301. [Google Scholar] [CrossRef]
- Lohsoontorn, P.; Brett, D.J.L.; Brandon, N.P. Thermodynamic predictions of the impact of fuel composition on the propensity of sulfur to interact with Ni and ceria-based anodes for solid oxide fuel cells. J. Power Sources 2008, 175, 60–67. [Google Scholar] [CrossRef]
- Xu, C.; Zondlo, J.W.; Gong, M.; Elizalde-Blancas, F.; Liu, X.; Celik, I.B. Tolerance tests of H2S-laden biogas fuel on solid oxide fuel cells. J. Power Sources 2010, 195, 4583–4592. [Google Scholar] [CrossRef]
- Shiratori, Y.; Oshima, T.; Sasaki, K. Feasibility of direct-biogas SOFC. Int. J. Hydrogen Energy 2008, 33, 6316–6321. [Google Scholar] [CrossRef]
- Grgicak, C.M.; Green, R.G.; Giorgi, J.B. SOFC anodes for direct oxidation of hydrogen and methane fuels containing H2S. J. Power Sources 2008, 179, 317–328. [Google Scholar] [CrossRef]
- Marianowski, L.G.; Anderson, G.L.; Camara, E.H. Use of Sulfur Containing Fuel in Molten Carbonate Fuel Cells. US Patent 5071718 10 December 1991. [Google Scholar]
- Bartholomew, C.H. Carbon Deposition in Steam Reforming and Methanation. Catal. Rev. 1982, 24, 67–112. [Google Scholar] [CrossRef]
- Liu, M.; Wei, G.; Luo, J.; Sanger, A.R.; Chuang, K.T. Use of metal sulfides as anode catalysts in H2S-Air SOFCs. J. Electrochem. Soc. 2003, 150, A1025–A1029. [Google Scholar] [CrossRef]
- Mukherjee, J.; Linic, S. First-Principles Investigations of Electrochemical Oxidation of Hydrogen at Solid Oxide Fuel Cell Operating Conditions. J. Electrochem. Soc. 2007, 154, B919–B924. [Google Scholar] [CrossRef]
- Jia, L.; Wang, X.; Hua, B.; Li, W.; Chi, B.; Pu, J.; Yuan, S. Computational analysis of atomic C and S adsorption on Ni, Cu, and Ni-Cu SOFC anode surfaces. Int. J. Hydrogen Energy. 2012, 37, 11941–11945. [Google Scholar] [CrossRef]
- Lang, M.; Bohn, C.; Henke, M.; Schiller, G.; Willich, C.; Hauler, F. Understanding the current-voltage behavior of high temperature solid oxide fuel cell stacks. J. Electrochem. Soc. 2017, 164, F1460–F1470. [Google Scholar] [CrossRef]
- Subhash, C.S.; Kendall, K. (Eds.) High-Temperature Solid Oxide Fuel Cells Fundamentals, Design And Applications; Elsevier Science: Oxford, UK, 2003; p. 272. [Google Scholar]
- Jamil, Z.; Ruiz-Trejo, E.; Boldrin, P.; Brandon, N.P. Anode fabrication for solid oxide fuel cells: Electroless and electrodeposition of nickel and silver into doped ceria scaffolds. Int. J. Hydrogen Energy 2016, 41, 9627–9637. [Google Scholar] [CrossRef] [Green Version]
- Price, R.; Grolig, J.G.; Mai, A.; Irvine, J.T.S. Evaluating sulfur-tolerance of metal/Ce0.80Gd0.20O1.90 co-impregnated La0.20Sr0.25Ca0.45TiO3 anodes for solid oxide fuel cells. Solid State Ionics 2020, 347, 115254. [Google Scholar] [CrossRef]
- Jais, A.A.; Ali, S.A.M.; Anwar, M.; Somalu, M.R.; Muchtar, A.; Isahak, W.N.R.W.; Baharudin, N.A.; Lim, K.L.; Brandon, N.P. Performance of Ni/10Sc1CeSZ anode synthesized by glycine nitrate process assisted by microwave heating in a solid oxide fuel cell fueled with hydrogen or methane. J. Solid State Electrochem. 2020, 24, 711–722. [Google Scholar] [CrossRef]
- Lay, E.; Gauthier, G.; Rosini, S.; Savaniu, C.; Irvine, J.T.S. Ce-substituted LSCM as new anode material for SOFC operating in dry methane. Solid State Ionics 2008, 179, 1562–1566. [Google Scholar] [CrossRef]
- Kim, G.; Corre, G.; Irvine, J.T.S.; Vohs, J.M.; Gorte, R.J. Engineering composite oxide SOFC anodes for efficient oxidation of methane. Electrochem. Solid-State Lett. 2008, 11, B16–B19. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Wang, S.; Wang, Z.; Ye, X.; Wen, T.; Huang, F. Anode performance of LST-xCeO2 for solid oxide fuel cells. J. Power Sources 2008, 183, 114–117. [Google Scholar] [CrossRef]
- Lo Faro, M.; La Rosa, D.; Nicotera, I.; Antonucci, V.; Aricò, A.S. Electrochemical investigation of a propane-fed solid oxide fuel cell based on a composite Ni-perovskite anode catalyst. Appl. Catal. B Environ. 2009, 89, 49–57. [Google Scholar] [CrossRef]
- Raj, E.S.; Irvine, J.T.I. Synthesis and characterization of (Pr0.75 Sr0.25)1−xCr0.5 Mn0.5O3−δ as anode for SOFCs. Solid State Ion. 2010, 180, 1683–1689. [Google Scholar] [CrossRef]
- Fuerte, A.; Valenzuela, R.X.; Escudero, M.J.; Daza, L. Effect of cobalt incorporation in copper-ceria based anodes for hydrocarbon utilisation in intermediate temperature solid oxide fuel cells. J. Power Sources 2011, 196, 4324–4331. [Google Scholar] [CrossRef]
- Jin, C.; Yang, C.; Zheng, H.; Chen, F. Intermediate temperature solid oxide fuel cells with Cu1.3Mn1.7O4 internal reforming layer. J. Power Sources 2012, 201, 66–71. [Google Scholar] [CrossRef]
- Wang, H.K.; Alfred, J.S.; Thangadurai, V. Trends in electrode development for next generation solid oxide fuel cells. J. Mater. Chem. A 2016, 4, 17913. [Google Scholar]
- Prasad, D.H.; Ji, H.I.; Kim, H.R.; Son, J.W.; Kim, B.K.; Lee, H.W.; Lee, J.H. Effect of nickel nano-particle sintering on methane reforming activity of Ni-CGO cermet anodes for internal steam reforming SOFCs. Appl. Catal. B Environ. 2011, 101, 531–539. [Google Scholar] [CrossRef]
- Osinkin, D.A.; Bogdanovich, N.M.; Beresnev, S.M.; Zhuravlev, V.D. High-performance anode-supported solid oxide fuel cell with impregnated electrodes. J. Power Sources 2015, 288, 20–25. [Google Scholar] [CrossRef]
- Razpotnik, T.; Ma, J. Synthesis of nickel oxide/zirconia powders via a modified Pechini method. J. European Ceram. Soc. 2007, 27, 1405–1410. [Google Scholar] [CrossRef]
- Cela, B.; De Macedo, D.A.; De Souza, G.L.; Martinelli, A.E.; Rubens, M.; Paskocimas, C.A. NiO–CGO in situ nanocomposite attainment: One step synthesis. J. Power Sources 2011, 196, 2539–2544. [Google Scholar] [CrossRef] [Green Version]
- Suciu, C.; Hoffmann, A.C.; Dorolti, E.; Tetean, R. NiO/YSZ nanoparticles obtained by new sol-gel route. Chem. Eng. J. 2008, 140, 586–592. [Google Scholar] [CrossRef]
- Li, S.; Guo, R.; Li, J.; Chen, Y.; Liu, W. Synthesis of NiO–ZrO2 powders for solid oxide fuel cells. Ceram. Int. 2003, 29, 883–886. [Google Scholar] [CrossRef]
- Grgicak, C.M.; Green, R.G.; Du, W.F.; Giorgi, J.B. Synthesis and characterization of NiO-YSZ anode materials: Precipitation, calcination, and the effects on sintering. J. Am. Ceram. Soc. 2005, 88, 3081–3087. [Google Scholar] [CrossRef]
- Yatsimirskii, K.; Volchenskova, I. Characteristics of chemical bonding in aquoamino complexes of nickel (II) determined by there absorption spectra. Teor. Eksp. Khim. 1967, 3, 17–23. [Google Scholar]
- Lin, J.-D.; Hsieh, T.-H. Preparation and structure development of NiO/YSZ nanocomposite powders by urea hydrolysis. Mater. Chem. Phys. 2010, 119, 553–561. [Google Scholar] [CrossRef]
- GooLee, J.; Jeon, O.S.; Hwang, H.J.; Jang, J.; Lee, Y.; Hyun, S.H.; Shul, Y.G. Durable and high-performance direct-methane fuel cells with coke-tolerant ceria-coated Ni catalysts at reduced temperatures. Electrochim. Acta 2016, 191, 677–686. [Google Scholar]
- Lee, D.; Myung, J.; Tan, J.; Hyun, S.; John, T.S.I.; Kim, J.; Moon, J. Direct methane solid oxide fuel cells based on catalytic partial oxidation enabling complete coking tolerance of Ni-based anodes. J. Power Sources 2017, 345, 30–40. [Google Scholar] [CrossRef]
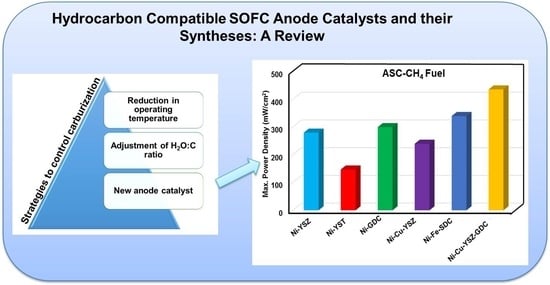
- Senthil Kumar, S.; Jayaram, V.; Aruna, S.T. Enhanced power density in hydrocarbon compatible anode supported solid oxide fuel cell. Nanomater. Energy 2021, 10, 118–127. [Google Scholar] [CrossRef]
- Moritz, L.W.; Wilhelm, M.; Jin, L.; Breuer, U.; Dittmann, R.; Waser, R.; Guillon, O.; Lenser, C.; Gunkel, F. Exsolution of embedded nanoparticles in defect engineered perovskite layers. ACS Nano 2021, 15, 4546–4560. [Google Scholar]
- Sun, Y.; Zhang, Y.; Chen, J.; Li, J.-H.; Zhu, Y.-T.; Zeng, Y.-M.; Amirkhiz, B.S.; Hua, B.; Luo, J.-L. New Opportunity for in Situ Exsolution of Metallic Nanoparticles on Perovskite Parent. Nano Lett. 2016, 16, 5303–5309. [Google Scholar] [CrossRef]
- Madsen, B.D.; Kobsiriphat, W.; Wang, Y.; Marks, L.D.; Barnett, S. SOFC Anode Performance Enhancement through Precipitation of Nanoscale Catalysts. ECS Trans. 2007, 7, 1339–1348. [Google Scholar] [CrossRef]
- Bian, L.; Duan, C.; Wang, L.; O’Hayre, R.; Cheng, J.; Chou, K.-C. Ce-doped La0.7Sr0.3Fe0.9Ni0.1O3-δ symmetrical electrodes for high performance direct hydrocarbon solid oxide fuel cells. J. Mater. Chem. A 2017, 5, 15253–15259. [Google Scholar] [CrossRef]
- Faro, M.L.; Zignani, S.C.; Aricò, A.S. Lanthanum ferrites-based exsolved perovskites as fuel-flexible anode for solid oxide fuel cells. Materials 2020, 13, 3231. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, L.; Chi, B.; Pu, J.; Li, J. In situ exsolved Ni-decorated Ba(Ce0.9Y0.1)0.8Ni0.2O3−δ perovskite as carbon-resistant composite anode for hydrocarbon-fueled solid oxide fuel cells. ACS Omega 2019, 4, 21494–21499. [Google Scholar] [CrossRef] [Green Version]
- Qin, M.; Tan, T.; Li, K.; Wang, Z.; Yang, H.; Liu, Z.; Zhou, M.; Liu, T.; Yang, C.; Liu, M. In-situ exsolved FeRu alloy nanoparticles on Ruddleson-Popper oxides for direct hydrocarbon fuel solid oxide fuel cells. Int. J. Hydrogen Energy 2020, 45, 21464–21472. [Google Scholar] [CrossRef]
- Xi, X.; Cao, Z.-S.; Shen, X.-Q.; Lu, Y.; Li, J.; Luo, J.-L.; Fu, X.-Z. In situ embedding of CoFe nanocatalysts into Sr3FeMoO7 matrix as high-performance anode materials for solid oxide fuel cells. J. Power Sources 2020, 459, 228071. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, L.; Li, J.; Chi, B.; Pu, J.; Li, J. High-performance Ni in-situ exsolved Ba(Ce0.9Y0.1)0.8Ni0.2O3-δ/Gd0.1Ce0.9O1.95 composite anode for SOFC with long-term stability in methane fuel. Compos. B Eng. 2020, 193, 108033. [Google Scholar] [CrossRef]
- Qin, M.; Xiao, Y.; Yang, H.; Tan, T.; Wang, Z.; Fan, X.; Yang, C. Ru/Nb co-doped perovskite anode: Achieving good coking resistance in hydrocarbon fuels via core-shell nanocatalysts exsolution. Appl. Catal. B Environ. 2021, 299, 120613. [Google Scholar] [CrossRef]
- Sholklapper, T.Z.; Kurokawa, H.; Jacobson, C.P.; Visco, S.J.; De Jonghe, L.C. Nanostructured solid oxide fuel cell electrodes. Nano Lett. 2007, 7, 2136–2141. [Google Scholar] [CrossRef] [Green Version]
- Moon, J.W.; Lee, H.L.; Kim, J.D.; Kim, G.D.; Lee, D.A.; Lee, H.W. Preparation of ZrO2-coated NiO powder using surface-induced coating. Mater. Lett. 1999, 38, 214–220. [Google Scholar] [CrossRef]
- Iwanschitz, B.; Mai, A.; Holzer, L.; Hocker, T.; Schütze, M. Degradation of Ni-cermet anodes in solid oxide fuel cells. In Proceedings of the 9th European Solid Oxide Fuel Cell Forum EFCF, Lucerne, Switzerland, 26–29 June 2010; pp. 7–61. [Google Scholar]
- Qiao, J.; Sun, K.; Zhang, N.; Sun, B.; Kong, J.; Zhou, D. Ni/YSZ and Ni–CeO2/YSZ anodes prepared by impregnation for solid oxide fuel cells. J. Power Sources 2007, 169, 253–258. [Google Scholar] [CrossRef]
- Li, W.; Lü, Z.; Zhu, X.; Guan, B.; Wei, B.; Guan, C.; Su, W. Effect of adding urea on performance of Cu/CeO2/yttria-stabilized zirconia anodes for solid oxide fuel cells prepared by impregnation method. Electrochim. Acta 2011, 56, 2230–2236. [Google Scholar] [CrossRef]
- Sadykov, V.; Mezentseva, N.; Alikina, G.; Bunina, R.; Pelipenko, V.; Lukashevich, A.; Tikhov, S.; Usoltsev, V. Nanocomposite catalysts for internal steam reforming of methane and biofuels in solid oxide fuel cells: Design and performance. Catal. Today 2009, 146, 132–140. [Google Scholar] [CrossRef]
- Zhan, Z.; Bierschenk, D.M.; Cronin, J.S.; Barnett, S.A. A reduced temperature solid oxide fuel cell with nanostructured anodes. Energy Environ. Sci. 2011, 4, 3951–3954. [Google Scholar] [CrossRef]
- Jiang, S.P. A review of wet impregnation—An alternative method for the fabrication of high performance and nano-structured electrodes of solid oxide fuel cells. Mater. Sci. Eng. 2006, A 418, 199–210. [Google Scholar] [CrossRef]
- Jiang, S.P.; Wang, W. Fabrication and performance of GDC-impregnated (La , Sr) MnO3 cathodes for intermediate temperature solid oxide fuel cells. J. Electrochem. Soc. 2005, 152, A1398–A1408. [Google Scholar] [CrossRef]
- Park, S.; Gorte, R.J.; Vohs, J.M. Tape cast solid-oxide fuel cells for the direct oxidation of hydrocarbons. J. Electrochem. Soc. 2001, 148, A443–A447. [Google Scholar] [CrossRef]
- Uchida, H.; Suzuki, S.; Watanabe, M. High Performance Electrode for Medium-Temperature Solid Oxide Fuel Cells: Mixed Conducting Ceria-Based Anode with Highly Dispersed Ni Electrocatalysts. Electrochem. Solid-State Lett. 2003, 6, A174–A177. [Google Scholar] [CrossRef]
- Simner, S.P.; Bonnett, J.F.; Canfield, N.L.; Meinhardt, K.D.; Shelton, J.P.; Sprenkle, V.L.; Stevenson, J.W. Development of lanthanum ferrite SOFC cathodes. J. Power Sources 2003, 113, 1–10. [Google Scholar] [CrossRef]
- Okawa, Y.; Matsumoto, T.; Doi, T.; Hirata, Y. Thermal stability of nanometer-sized NiO and Sm-doped ceria powders. J. Mater. Res. 2002, 17, 2266–2274. [Google Scholar] [CrossRef]
- McIntosh, S.; Vohs, J.M.; Gorte, R.J. Effect of Precious-Metal Dopants on SOFC Anodes for Direct Utilization of Hydrocarbons. Electrochem. Solid-State Lett. 2003, 6, A240. [Google Scholar] [CrossRef]
- Jiang, S.P. Issues on development of (La,Sr)MnO3 cathode for solid oxide fuel cells. J. Power Sources 2003, 124, 390–402. [Google Scholar] [CrossRef]
- Venâncio, S.A.; Sarruf, B.J.M.; Gomes, G.G.; Miranda, P.E.V. Multifunctional macroporous solid oxide fuel cell anode with active nanosized ceramic electrocatalyst. Int. J. Hydrogen Energy 2020, 45, 5501–5511. [Google Scholar] [CrossRef]
- Fukui, T.; Murata, K.; Ohara, S.; Abe, H.; Naito, M.; Nogi, K. Morphology control of Ni—YSZ cermet anode for lower temperature operation of SOFCs. J. Power Sources 2004, 125, 17–21. [Google Scholar] [CrossRef]
- Chou, C.-S.; Yang, R.-Y.; Yeh, C.-K.; Lin, Y.-J. Preparation of TiO2/Nano-metal composite particles and their applications in dye-sensitized solar cells. Powder Technol. 2009, 194, 95–105. [Google Scholar] [CrossRef]
- Misono, T.; Murata, K.; Yin, J.; Fukui, T. Morphology control of Ni-GDC cermet anode for lower temperature SOFC. ECS Trans. 2007, 7, 1355–1361. [Google Scholar] [CrossRef]
- Mukhopadhyay, M.; Mukhopadhyay, J.; Basu, R.N. Functional Anode Materials for Solid Oxide Fuel Cell—A Review. Trans. Ind. Ceram. Soc. 2013, 72, 145–168. [Google Scholar] [CrossRef]
- Rahman, A.H.M.E.; Kim, J.; Lee, K.; Lee, B. Microstructure characterization and electrical conductivity of electroless nano Ni coated 8YSZ cermets. Surf. Coat. Technol. 2008, 202, 2182–2188. [Google Scholar] [CrossRef]
- Beckel, D.; Bieberle-Hütter, A.; Harvey, A.; Infortuna, A.; Muecke, U.P.; Prestat, M.; Rupp, J.L.M.; Gauckler, L.J. Thin films for micro solid oxide fuel cells. J. Power Sources 2007, 173, 325–345. [Google Scholar] [CrossRef]
- Lao, G.J.; Hertz, J.; Tuller, H.; Shao-Horn, Y. Microstructural Features of RF-sputtered SOFC Anode and Electrolyte Materials. J. Electroceram. 2004, 13, 691–695. [Google Scholar] [CrossRef]
- Jou, S.; Wu, T.-H. Thin porous Ni–YSZ films as anodes for a solid oxide fuel cell. J. Phys. Chem. Solids. 2008, 69, 2804–2812. [Google Scholar] [CrossRef]
- Rezugina, E.; Thomann, A.L.; Hidalgo, H.; Brault, P.; Dolique, V.; Tessier, Y. Ni-YSZ films deposited by reactive magnetron sputtering for SOFC applications. Surf. Coat. Technol. 2010, 204, 2376–2380. [Google Scholar] [CrossRef] [Green Version]
- Klotz, D.; Butz, B.; Leonide, A.; Hayd, J.; Gerthsen, D.; Ivers-Tiffée, E. Performance enhancement of SOFC anode through electrochemically induced Ni/YSZ nanostructures. J. Electrochem. Soc. 2011, 158, B587–B595. [Google Scholar] [CrossRef]
- Alinina, E.; Pikalova, E. Opportunities, challenges and prospects for electrodeposition of thin-film functional layers in solid oxide fuel cell technology. Materials 2021, 14, 5584. [Google Scholar] [CrossRef]
- Hu, S.; Li, W.; Finklea, H.; Liu, X. A review of electrophoretic deposition of metal oxides and its application in solid oxide fuel cells. Adv. Colloid Interface Sci. 2020, 276, 102102. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Kalinina, E.G. Electrophoretic deposition in the solid oxide fuel cell technology: Fundamentals and recent advances. Renew. Sustain. Energy Rev. 2019, 116, 109440. [Google Scholar] [CrossRef]
- Pikalova, E.Y.U.; Kalinina, E.G. Place of electrophoretic deposition among thin-film methods adapted to the solid oxide fuel cell technology: A short review. Int. J. Energy Prod. Manag. 2019, 4, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Salehzadeh, D.; Torabi, M.; Sadeghian, Z.; Marashi, P. A multiscale-architecture solid oxide fuel cell fabricated by electrophoretic deposition technique. J. Alloys Compd. 2020, 830, 154654. [Google Scholar] [CrossRef]
- Melnik, J.; Fu, X.Z.; Luo, J.L.; Sanger, A.R.; Chuanga, K.T.; Yang, Q.M. Ceria and copper/ceria functional coatings for electrochemical applications: Materials preparation and characterization. J. Power Sources 2010, 195, 2189–2195. [Google Scholar] [CrossRef]
- Dos Santos-Gómez, L.; Zamudio-García, J.; Porras-Vázquez, J.M.; Losilla, E.R.; Marrero-López, D. Recent progress in nanostructured electrodes for solid oxide fuel cells deposited by spray pyrolysis. J. Power Sources 2021, 507, 230277. [Google Scholar] [CrossRef]
- Shih, S.-J.; Widagdyo, D.R. Preparation of mesoporous SrTiO3 particles by spray pyrolysis method. J. Nanosci. Nanotechnol. 2017, 17, 3557–3565. [Google Scholar] [CrossRef]
- Lim, C.-H.; Lee, K.-T. Characterization of core-shell structured Ni@GDC anode materials synthesized by ultrasonic spray pyrolysis for solid oxide fuel cells. Ceram. Int. 2016, 42, 13715–13722. [Google Scholar] [CrossRef]
- Lim, C.-H.; Lee, K.-T. Characterization of spherical NiO-YSZ anode composites for solid oxide fuel cells synthesized by ultrasonic spray pyrolysis. J. Korean Ceram. Soc. 2014, 51, 243–247. [Google Scholar] [CrossRef] [Green Version]
- Hashigami, S.; Yoshida, H.; Ueno, D.; Kawano, M.; Inagaki, T. Improvement of the redox durability of Ni-gadolinia doped ceria anodes due to the use of the composite particles prepared by spray pyrolysis method. J. Power Sources 2014, 248, 190–195. [Google Scholar] [CrossRef]
- Genji, K.; Myoujin, K.; Kodera, T.; Ogihara, T. Synthesis and electrical properties of La doped SrTiO3 powders by ultrasonic spray pyrolysis. Key Eng. Mater. 2014, 582, 115–118. [Google Scholar] [CrossRef]
- Dos Santos-Gómez, L.; Porras-Vázquez, J.M.; Losilla, E.R.; Marrero-López, D. Ti-doped SrFeO3 nanostructured electrodes for symmetric solid oxide fuel cells. RSC Adv. 2015, 5, 107889–107895. [Google Scholar] [CrossRef]
- Tanveer, W.H.; Iwai, H.; Yu, W.; Pandiyan, A.; Ji, S.; Lee, Y.H.; Lee, Y.; Yaqoob, K.; Cho, G.Y.; Cha, S.W. Experimentation and modelling of nanostructured nickel cermet anodes for submicron SOFCs fuelled indirectly by industrial waste carbon. J. Mater. Chem. A 2018, 6, 11169–11179. [Google Scholar] [CrossRef]
- Iwata, T. Characterization of Ni-YSZ Anode degradation for substrate-type solid oxide fuel cells. J. Electrochem. Soc. 1996, 143, 1521–1525. [Google Scholar] [CrossRef]
- Iwanschitz, B.; Sfeir, J.; Mai, A.; Schütze, M. Degradation of SOFC Anodes upon Redox Cycling: A Comparison Between Ni/YSZ and Ni/CGO. J. Electrochem. Soc. 2010, 157, B269. [Google Scholar] [CrossRef]
- Costamagna, P.; Costa, P.; Antonucci, V. Micro-modelling of solid oxide fuel cell electrodes. Electrochim. Acta 1998, 43, 375–394. [Google Scholar] [CrossRef]
- Klemensø, T.; Appel, C.C.; Mogensen, M. In situ observations of microstructural changes in SOFC anodes during redox cycling. Electrochem. Solid-State Lett. 2006, 9, A403–A407. [Google Scholar] [CrossRef] [Green Version]
- Pihlatie, M.H.; Frandsen, H.L.; Kaiser, A.; Mogensen, M. Continuum mechanics simulations of NiO/Ni–YSZ composites during reduction and re-oxidation. J. Power Sources 2010, 195, 2677–2690. [Google Scholar] [CrossRef]
- Gubner, A.; Landes, H.; Metzger, J.; Seeg, H.; Stübner, R. Investigation into the Degradation of the Cermet Anode of a Solid Oxide Fuel Cell. In Proceedings of the Fifth International Symposium on Solid Oxide Fuel Cells (SOFC-V), Aachen, Germany, 2–5 June 1997; Stimming, U., Singhal, S.C., Tagawa, H., Lehnert, W., Eds.; The Electrochemical Society: Pennington, NJ, USA, 1997; pp. 844–850. [Google Scholar]
- Simwonis, D.; Tietz, F.; Stöver, D. Nickel coarsening in annealed Ni/8YSZ anode substrates for solid oxide fuel cells. Solid State Ion. 2000, 132, 241–251. [Google Scholar] [CrossRef]
- Vassen, R.; Simwonis, D.; Stöver, D. Modelling of the agglomeration of Ni-particles in anodes of solid oxide fuel cells. J. Mater. Sci. 2001, 36, 147–151. [Google Scholar] [CrossRef]
- Jiang, S.P. Sintering behavior of Ni/Y2O3-ZrO2 cermet electrodes of solid oxide fuel cells. J. Mater. Sci. 2003, 38, 3775–3782. [Google Scholar] [CrossRef]
- Faes, A.; Hessler-Wyser, A.; Presvytes, D.; Vayenas, C.G. Nickel–zirconia anode degradation and triple phase boundary quantification from microstructural analysis. Fuel Cells 2009, 9, 841–851. [Google Scholar] [CrossRef]
- Holzer, L.; Iwanschitz, B.; Hocker, T.; Münch, B.; Prestat, M.; Wiedenmann, D.; Vogt, U.; Holtappels, P. Microstructure degradation of cermet anodes for solid oxide fuel cells: Quantification of nickel grain growth in dry and in humid atmospheres. J. Power Sources 2011, 196, 1279–1294. [Google Scholar] [CrossRef]
- Iwanschitz, B.; Holzer, L.; Mai, A.; Schütze, M. Nickel agglomeration in solid oxide fuel cells: The influence of temperature. Solid State Ion. 2012, 211, 69–73. [Google Scholar] [CrossRef]
























| Types of Reactions | Reactions Involved | Temp. Range (°C) | Advantages | Limitations |
|---|---|---|---|---|
| Steam Reforming | (syngas) (endothermic) (exothermic) | 700 | Suitable for external reformers | Reforming fuel inside SOFC anode (internal steam reforming) will affect the performance; more steam results in low OCV in SOFC due to fuel dilution |
| Autothermal Reforming (ATR) | (exothermic) | 950–1060 | Reactor is compact in design | Reaction pressure is in the range 30–50 bar |
| Partial Oxidation (POX) | Thermal partial oxidation (TPOX) (exothermic) Catalytic partial oxidation (CPOX) (with catalyst) | 1200 | CPOX reaction utilizes a catalyst to reduce the reaction temperature to around 800–900 °C | CPOX is suitable only for low sulfur (<50 ppm) fuel as catalysts are much more prone to sulfur poisoning.CPOX has seldom been used in SOFC anodes, as the pO2 in the chamber must be sustained <10−18 atm to trigger SOFC operation |
| Direct Methane (DM) Oxidation | >750 | DM-SOFCs possess maximum catalytic activity for complete oxidation of methane | Oxidation of Ni |
| Anode | Electrolyte | Cathode | Fuel | Temp. °C | Power Density (Wcm−2) | Stability | Ref. |
|---|---|---|---|---|---|---|---|
| (PrBa)0.95(Fe0.9Nb0.1)2O5+δ oxide | LSGM | PrBaCo2O5+δ | H2 wet CH4 dry CH4 | 800 | 1.05 0.64 0.57 | Highly coking resistant in dry CH4 for 200 h | [229] |
| MgO-modified Ni–SDC | LSGM | SCCO-SDC | Humidified CH4 | 800 | 0.714 | Excellent long-term stability for 330 h | [230] |
| Ni–SDC | SDC | SSC-SDC | Liquid methanol | 650 | 0.698 | No degradation during 60 h long-term testing | [231] |
| Li-modified Ni–SDC Na-modified Ni–SDC | LSGM | SCCO-SDC | Wet CH4 | 800 | 0.212 0.231 | Stability up to 70 h | [232] |
| MoO2-based anode | YSZ | LSM | n-dodecane | 850 | Initially 0.34; after optimizing porosity of anode = 2.5 | Highly coke resistant with improved long-term stability | [233] |
| Ni–GDC with 0.5 wt% Sn catalyst | GDC | LSCF-GDC | Dry CH4 | 650 | 0.93 | Operates for over 40 h without degradation | [234] |
| MoO2 porous thin film deposited on Ni–YSZ | YSZ | LSM | Mixture of n-dodecane and air | 750 | >4.0 | 24 h Highly coking resistant | [235] |
| Ni-Fe-LSGM | LSGM | SSC | CH4 | 800 | 0.48 | Good stability as no coke deposition after 15 h | [236] |
| Ni-infiltrated porous GDC scaffolds | GDC | LSCF | Wet CH4 | 600 | 0.125 | Stable operation for 24 h | [237] |
| Layered PrBaMn2O5+δ anode with PrBaMn2O5+δ and Co–Fe catalyst | LSGM | NdBa0.5Sr0.5 Co1.5 Fe0.5 O5+δ–Ce0.9 Gd0.1 O2−δ (NBSCF50-GDC) | C3H8 | 700 | 1.32 | Long-term stability tested for 500 h | [140] |
| Ni–YSZ | YSZ | LSM-YSZ |
| Anode | Configuration | ASR (Ωcm2) | Temp. (°C) | Fuel | Power Density mW cm−2 | Ref. |
|---|---|---|---|---|---|---|
| Ni/Ag/GDC | YSZ/LSCF-GDC | 1.12 | 750 | Syngas | 33 | [271] |
| La0.20Sr0.25Ca0.45TiO3 decorated with CGO coating and nanoparticles of Ni, Pt, or Rh. | 6ScSZ/LSM-YSZ | 0.78 | 850 | Syngas + 8 ppm H2S | - | [272] |
| Ni/10Sc1CeSZ | 10Sc1CeSZ symmetrical cell | 24.3 | 800 | CH4 | - | [273] |
| La0.65 Ce0.1Sr0.25Cr0.5 Mn0.5O3−δ | ESC-YSZ | 1.6 | 900 | CH4 | - | [274] |
| Ni- Ce0.9Sr0.1 Cr0.5V0.5 O3-YSZ | ESC-YSZ | 5.9 | 850 | H2S-CH4 | 25 | [251] |
| La0.8 Sr0.2ScxMn1−xO3–GDC-Pd | Three-layer ESC infiltration | 0.4 | 700 | CH4 | 341 | [139] |
| La0.75Sr0.25Cr0.5Mn0.5O3−δ | ESC-YSZ-symmetrical cell | 0.613 | 950 | CH4 | 347 | [74] |
| LSCM-0.5%Pd-5%CeO2 | Three-layer ESC infiltration | 0.14 | 800 | CH4 | 710 | [275] |
| La0.75Sr0.25Cr0.5Mn0.5 O3−δ | ESC | 2.3 | 800 | Ethanol | 101 | [72] |
| (La0.75Sr0.25)Cr0.5 Mn0.5 O3−δ | ESC-YSZ-Symmetric | 0.3 | 900 | CH4 | 230 | [73] |
| La0.8Sr0.2ScxMn1−xO3–GDC-Pd | Three-layer ES infiltration | 0.4 | 700 | CH4 | 341 | [163] |
| Sr0.6La0.4TiO3-50% CeO2 | ESYSZ | 2 | 900 | CH4 | 139.6 | [276] |
| La0.2Sr0.7TiO3-GDC-Cu | Three-layer ESC infiltration | 0.15 | 750 | CH4 | 540 | [100] |
| 10%Ni-LSFC | ES-CGO | 0.33 | 800 | Propane | 421 | [277] |
| (Pr0.75Sr0.25)1−xCr0.5 Mn0.5O3−δ | ES-YSZ | 3.52 | 910 | CH4 | 18 | [278] |
| 1:1 Co-Cu-CeO2 | ESC-YSZ | 3.72 | 800 | CH4 | 67 | [279] |
| 50:50 CoCu-CeO2-YSZ | 3-layer-YSZ | 0.8 | 800 | CH4 | 250 | [221] |
| Cu1.3Mn1.7O4 on Ni–SDC | ASC-SDC | 0.120 | 700 | CH4 | 375 | [280] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senthil Kumar, S.; Aruna, S.T. Hydrocarbon Compatible SOFC Anode Catalysts and Their Syntheses: A Review. Sustain. Chem. 2021, 2, 707-763. https://doi.org/10.3390/suschem2040039
Senthil Kumar S, Aruna ST. Hydrocarbon Compatible SOFC Anode Catalysts and Their Syntheses: A Review. Sustainable Chemistry. 2021; 2(4):707-763. https://doi.org/10.3390/suschem2040039
Chicago/Turabian StyleSenthil Kumar, Selvaraj, and Singanahally ThippaReddy Aruna. 2021. "Hydrocarbon Compatible SOFC Anode Catalysts and Their Syntheses: A Review" Sustainable Chemistry 2, no. 4: 707-763. https://doi.org/10.3390/suschem2040039