What Is Psychological Spin? A Thermodynamic Framework for Emotions and Social Behavior
Abstract
:1. Introduction
2. Mental Unity
3. Discussion
3.1. Mental Homeostasis
3.2. The Thermodynamic Analysis of the Evoked Cycle
3.3. Quantum Cognition
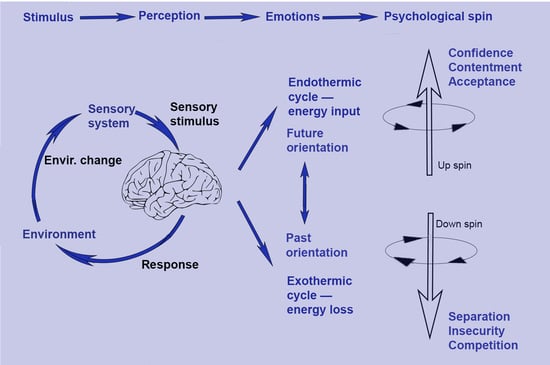
3.4. Psychological Spin
3.5. The Interpretation of Spinor Psychology
3.6. The Psychological Down Spin
3.7. The Psychological Up Spin
4. Discussion and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitko, A.; Fischer, J. When it all falls down: The relationship between intuitive physics and spatial cognition. Cogn. Res. Princ. Implic. 2020, 5, 24. [Google Scholar] [CrossRef]
- Perlovsky, L.I. Physics of the Mind. Front. Syst. Neurosci. 2016, 10, 84. [Google Scholar] [CrossRef]
- Dabaghian, Y. Through synapses to spatial memory maps via a topological model. Sci. Rep. 2019, 9, 572. [Google Scholar] [CrossRef]
- Herzog, M.H.; Drissi-Daoudi, L.; Doerig, A. All in Good Time: Long-Lasting Postdictive Effects Reveal Discrete Perception. Trends Cogn. Sci. 2020, 24, 826–837. [Google Scholar] [CrossRef]
- Fingelkurts, A.A.; Fingelkurts, A.A. Present Moment, Past, and Future: Mental Kaleidoscope. Front. Psychol. 2014, 5, 395. [Google Scholar] [CrossRef]
- Nes, A.; Sundberg, K.; Watzl, S. The perception/cognition distinction. Inquiry 2021, 66, 165–195. [Google Scholar] [CrossRef]
- Montemayor, C.; Haladjian, H.H. Perception and Cognition Are Largely Independent, but Still Affect Each Other in Systematic Ways: Arguments from Evolution and the Consciousness-Attention Dissociation. Front. Psychol. 2017, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, L.; Vallejos, L.; Cleare-Hoffman, H.P.; Rubin, S. Emotion, relationship, and meaning as core existential practice: Evidence-based foundations. J. Contemp. Psychother. Cut. Edge Mod. Dev. Psychother. 2015, 45, 11–20. [Google Scholar] [CrossRef]
- Xu, A.J.; Schwarz, N. How One Thing Leads to Another: Spillover Effects of Behavioral Mind-Sets. Curr. Dir. Psychol. Sci. 2018, 27, 51–55. [Google Scholar] [CrossRef]
- Surov, I.A. Quantum Core Affect. Color-Emotion Structure of Semantic Atom. Front. Psychol. 2022, 13, 838029. [Google Scholar] [CrossRef] [PubMed]
- Pothos, E.M.; Busemeyer, J.R. Quantum Cognition. Annu. Rev. Psychol. 2022, 73, 749–778. [Google Scholar] [CrossRef]
- Dennett, D.C. Facing up to the hard question of consciousness. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20170342. [Google Scholar] [CrossRef] [PubMed]
- Ohanian, H.C. What is spin? Am. J. Phys. 1986, 54, 500–505. [Google Scholar] [CrossRef]
- Reisenzein, R. What is a definition of emotion? And are emotions mental-behavioral processes? Soc. Sci. Inf. 2007, 46, 424–428. [Google Scholar] [CrossRef]
- O’Neill, J.; Schoth, A. The Mental Maxwell Relations: A Thermodynamic Allegory for Higher Brain Functions. Front. Neurosci. 2022, 16, 827888. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.J.J.; Jann, K.; Fan, C.; Qiao, Y.; Zang, Y.-F.; Lu, H.; Yang, Y. Neurophysiological Basis of Multi-Scale Entropy of Brain Complexity and Its Relationship With Functional Connectivity. Front. Neurosci. 2018, 12, 352. [Google Scholar] [CrossRef]
- Escobar, F.B.; Velasco, C.; Motoki, K.; Byrne, D.V.; Wang, Q.J. The temperature of emotions. PLoS ONE 2021, 16, e0252408. [Google Scholar] [CrossRef]
- Déli, E.; Kisvárday, Z. The thermodynamic brain and the evolution of intellect: The role of mental energy. Cogn. Neurodyn. 2020, 14, 743–756. [Google Scholar] [CrossRef]
- Deli, E.; Kisvarday, Z.; Peters, J.F. The Thermodynamics of Cognition: A Mathematical Treatment. Comput. Struct. Biotechnol. J. 2021, 19, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Suhaimi, N.S.; Mountstephens, J.; Teo, J. EEG-Based Emotion Recognition: A State-of-the-Art Review of Current Trends and Opportunities. Comput. Intell. Neurosci. 2020, 2020, 8875426. [Google Scholar] [CrossRef]
- Torres, E.P.; Torres, E.A.; Hernández-Álvarez, M.; Yoo, S.G. EEG-Based BCI Emotion Recognition: A Survey. Sensors 2020, 20, 5083. [Google Scholar] [CrossRef]
- Hesp, C.; Smith, R.; Parr, T.; Allen, M.; Friston, K.J.; Ramstead, M.J.D. Deeply Felt Affect: The Emergence of Valence in Deep Active Inference. Neural Comput. 2021, 33, 398–446. [Google Scholar] [CrossRef]
- Kao, F.C.; Wang, S.P.R.; Huang, C.H.; Chen, C.C.; Lin, Y.K. Brainwave analysis of positive and negative emotions. In Management, Information and Educational Engineering; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Gilchrist, J.C.; Nesberg, L.S. Need and perceptual change in need-related objects. J. Exp. Psychol. 1952, 44, 369–376. [Google Scholar] [CrossRef]
- Bruner, J.S.; Minturn, A.L. Perceptual Identification and Perceptual Organization. J. Gen. Psychol. 1955, 53, 21–28. [Google Scholar] [CrossRef]
- Babaev, O.; Chatain, C.P.; Krueger-Burg, D. Inhibition in the amygdala anxiety circuitry. Exp. Mol. Med. 2018, 50, 1–16. [Google Scholar] [CrossRef]
- Bechler, C.J.; Tormala, Z.L.; Rucker, D.D. Perceiving Attitude Change: How Qualitative Shifts Augment Change Perception. Stanford Graduate School of Business. J. Exp. Soc. Psychol. 2019, 83, 160–175. [Google Scholar] [CrossRef]
- Wang, Z. The Neurocognitive Correlates of Brain Entropy Estimated by Resting State fMRI-ScienceDirect. NeuroImage 2021, 232, 117893. [Google Scholar] [CrossRef]
- Fang, H.; He, B.; Fu, H.; Zhang, H.; Mo, Z.; Meng, L. A Surprising Source of Self-Motivation: Prior Competence Frustration Strengthens One’s Motivation to Win in Another Competence-Supportive Activity. Front. Hum. Neurosci. 2018, 12, 314. [Google Scholar] [CrossRef]
- Hutt, A.; Lefevre, J. Arousal Fluctuations Govern Oscillatory Transitions Between Dominant γ and α Occipital Activity During Eyes Open/Closed Conditions. Brain Topogr. 2022, 35, 108–120. [Google Scholar] [CrossRef]
- Scheidel, W. The Great Leveler: Violence and the History of Inequality from the Stone Age to the Twenty-First Century; Princeton University Press: Princeton, NJ, USA, 2018. [Google Scholar]
- Deli, E. Can the Fermionic Mind Hypothesis (FMH) Explain Consciousness? The Physics of Selfhood. Act. Nerv. Super. 2020, 62, 35–47. [Google Scholar] [CrossRef]
- Deli, E. Thermodynamic Implications of the Fermionic Mind Hypothesis. Act. Nerv. Super. 2020, 62, 96–103. [Google Scholar] [CrossRef]
- Bayne, T. Unity of consciousness. Scholarpedia 2009, 4, 7414. [Google Scholar] [CrossRef]
- Binocular Rivalry; MIT Press: Cambridge, MA, USA, 2005; pp. 1–373.
- Tong, F.; Meng, M.; Blake, R. Neural bases of binocular rivalry. Trends Cogn. Sci. 2006, 10, 502–511. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, D. Binaral rivalry between the nostrils and in the cortex. Curr. Biol. 2009, 19, 1561–1565. [Google Scholar] [CrossRef] [PubMed]
- Criscuolo, A.; Schwartze, M.; Kotz, S.A. Cognition through the lens of a body–brain dynamic system. Trends Neurosci. 2022, 45, 667–677. [Google Scholar] [CrossRef]
- Bastos, A.M.; Litvak, V.; Moran, R.; Bosman, C.A.; Fries, P.; Friston, K.J. A DCM study of spectral asymmetries in feedforward and feedback connections between visual areas V1 and V4 in the monkey. NeuroImage 2015, 108, 460–475. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhang, J.; Wu, J.; Mashour, G.A.; Hudetz, A.G. Temporal circuit of macroscale dynamic brain activity supports human consciousness. Sci. Adv. 2020, 6, eaaz0087. [Google Scholar] [CrossRef]
- Guterstam, A.; Abdulkarim, Z.; Ehrsson, H. Illusory Ownership of an Invisible Body Reduces Autonomic and Subjective Social Anxiety Responses. Sci. Rep. 2015, 5, 9831. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Spence, C. Assessing the role of the ‘unity assumption’ on multisensory integration: A review. Front. Psychol. 2017, 8, 445. [Google Scholar] [CrossRef]
- Min, J.; Nashiro, K.; Yoo, H.J.; Cho, C.; Nasseri, P.; Bachman, S.L.; Porat, S.; Thayer, J.F.; Chang, C.; Lee, T.-H.; et al. Emotion Downregulation Targets Interoceptive Brain Regions While Emotion Upregulation Targets Other Affective Brain Regions. J. Neurosci. Off. J. Soc. Neurosci. 2022, 42, 2973–2985. [Google Scholar] [CrossRef]
- Bányai, M.; Lazar, A.; Klein, L.; Klon-Lipok, J.; Stippinger, M.; Singer, W.; Orbán, G. Stimulus complexity shapes response correlations in primary visual cortex. Proc. Natl. Acad. Sci. USA 2019, 116, 2723–2732. [Google Scholar] [CrossRef] [PubMed]
- Singer, W. Recurrent Dynamics in the Cerebral Cortex: Integration of Sensory Evidence with Stored Knowledge. Proc. Natl. Acad. Sci. USA 2021, 118, e2101043118. [Google Scholar] [CrossRef] [PubMed]
- Stringer, C.; Pachitariu, M.; Steinmetz, N.; Carandini, M.; Harris, K.D. High-Dimensional Geometry of Population Responses in Visual Cortex. Nature 2019, 571, 361–365. [Google Scholar] [CrossRef]
- Northoff, G.; Tumati, S. “Average is good, extremes are bad”–Non-linear inverted U-shaped relationship between neural mechanisms and functionality of mental features. Neurosci. Biobehav. Rev. 2019, 104, 11–25. [Google Scholar] [CrossRef]
- Schoeller, F.; Perlovsky, L.I. Aesthetic Chills: Knowledge-Acquisition, Meaning-Making, and Aesthetic Emotions. Front. Psychol. 2016, 7, 1093. [Google Scholar] [CrossRef] [PubMed]
- Kolvoort, I.R.; Wainio-Theberge, S.; Wolff, A.; Northoff, G. Temporal integration as ‘common currency’ of brain and self-scale-free activity in resting-state EEG correlates with temporal delay effects on self-relatedness. Hum. Brain Mapp. 2020, 41, 4355–4374. [Google Scholar] [CrossRef]
- Wolff, A.; Di Giovanni, D.A.; Gómez-Pilar, J.; Nakao, T.; Huang, Z.; Longtin, A.; Northoff, G. The temporal signature of self: Temporal measures of resting-state EEG predict self-consciousness. Hum. Brain Mapp. 2019, 40, 789–803. [Google Scholar] [CrossRef]
- Kataoka, N.; Shima, Y.; Nakajima, K.; Nakamura, K. A central master driver of psychosocial stress responses in the rat. Science 2020, 367, 1105–1112. [Google Scholar] [CrossRef]
- Fadok, J.P.; Krabbe, S.; Markovic, M.; Courtin, J.; Xu, C.; Massi, L.; Botta, P.; Bylund, K.; Müller, C.; Kovacevic, A.; et al. A competitive inhibitory circuit for selection of active and passive fear responses. Nature 2017, 542, 96–100. [Google Scholar] [CrossRef]
- Manohar, S.G.; Muhammed, K.; Fallon, S.J.; Husain, M. Motivation dynamically increases noise resistance by internal feedback during movement. Neuropsychologia 2019, 123, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Caruana, F.; Gerbella, M.; Avanzini, P.; Gozzo, F.; Pelliccia, V.; Mai, R.; O Abdollahi, R.; Cardinale, F.; Sartori, I.; Russo, G.L.; et al. Motor and emotional behaviours elicited by electrical stimulation of the human cingulate cortex. Brain J. Neurol. 2018, 141, 3035–3051. [Google Scholar] [CrossRef] [PubMed]
- Ellard, K.K.; Barlow, D.H.; Whitfield-Gabrieli, S.; Gabrieli, J.D.E.; Deckersbach, T. Neural correlates of emotion acceptance vs worry or suppression in generalized anxiety disorder. Soc. Cogn. Affect. Neurosci. 2017, 12, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- Conant, R.C.; Ross Ashby, W. Every good regulator of a system must be a model of that system. Int. J. Syst. Sci. 1970, 1, 89–97. [Google Scholar] [CrossRef]
- Witkowski, P.P.; Park, S.A.; Boorman, E.D. Neural Mechanisms of Credit Assignment for Inferred Relationships in a Structured World. Neuron 2022, 110, 2680–2690. [Google Scholar] [CrossRef] [PubMed]
- Rowe, A.; Fitness, J. Understanding the Role of Negative Emotions in Adult Learning and Achievement: A Social Functional Perspective. Behav. Sci. 2018, 8, 27. [Google Scholar] [CrossRef]
- Shukuroglou, M.; Roseman, L.; Wall, M.; Nutt, D.; Kaelen, M.; Carhart-Harris, R. Changes in music-evoked emotion and ventral striatal functional connectivity after psilocybin therapy for depression. J. Psychopharmacol. 2023, 37, 70–79. [Google Scholar] [CrossRef]
- Shannon, C.E. Probability of Error for Optimal Codes in a Gaussian Channel. Bell Syst. Tech. J. 1959, 38, 611–656. [Google Scholar] [CrossRef]
- Deli, E.; Kisvarday, Z.; Peters, J.F. How the Brain Becomes the Mind: Can Thermodynamics Explain the Emergence and Nature of Emotions. Entropy 2022, 24, 1498. [Google Scholar] [CrossRef]
- Seebacher, F. Is Endothermy an Evolutionary By-Product? Trends Ecol. Evol. 2020, 35, 503–511. [Google Scholar] [CrossRef]
- Grigg, G.; Nowack, J.; Bicudo, J.E.P.W.; Bal, N.C.; Woodward, H.N.; Seymour, R.S. Whole-body endothermy: Ancient, homologous and widespread among the ancestors of mammals, birds and crocodylians. Biol. Rev. Camb. Philos. Soc. 2022, 97, 766–801. [Google Scholar] [CrossRef]
- Northoff, G. Is Our Brain an Open or Closed System? Prediction Model of Brain and World–Brain Relation. In The Spontaneous Brain: From the Mind-Body to the World-Brain Problem; MIT Press Scholarship Online|Oxford Academic: Cambridge, MA, USA, 2019; Available online: https://academic.oup.com/mit-press-scholarship-online/book/31439/chapter-abstract/289623328?redirectedFrom=fulltext (accessed on 13 November 2023).
- Deli, E.; Peters, J.F.; Tozzi, A. The thermodynamic analysis of neural computation. J. Neurosci. Clin Res. 2018, 3, 2. [Google Scholar]
- Zheng, J.; Wang, R.; Kong, W.; Zhang, J. A new patterns of self-organization activity of brain: Neural energy coding. Inf. Sci. Int. J. 2022, 608, 1747–1755. [Google Scholar] [CrossRef]
- Saxe, G.N.; Calderone, D.; Morales, L.J. Brain entropy and human intelligence: A resting-state fMRI study. PLoS ONE 2018, 13, e0191582. [Google Scholar] [CrossRef]
- Ryan, R.M.; Deci, E.L. Self-Determination Theory: Basic Psychological Needs in Motivation, Development, and Wellness; Guilford Press: New York, NY, USA; Available online: https://www.guilford.com/books/Self-Determination-Theory/Ryan-Deci/9781462538966 (accessed on 13 November 2023).
- Van Cappellen, P.; Rice, E.L.; Catalino, L.I.; Fredrickson, B.L. Positive affective processes underlie positive health behaviour change. Psychol. Health 2018, 33, 77–97. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Z.; Zhao, W.; Sun, Y.; Wen, D.; Xie, Y.; Liu, X.; Niu, H.; Han, Y. Decreased Resting-State Brain Signal Complexity in Patients with Mild Cognitive Impairment and Alzheimer’s Disease: A Multiscale Entropy Analysis. Biomed. Opt. Express. 2018, 9, 1916–1929. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, W. Implicit Attitudes and the Ability Argument. Philos. Stud. 2019, 176, 2961–2990. [Google Scholar] [CrossRef]
- Schubert, T.; Eloo, R.; Scharfen, J.; Morina, N. How Imagining Personal Future Scenarios Influences Affect: Systematic Review and Meta-Analysis. Clin. Psychol. Rev. 2020, 75, 101811. [Google Scholar] [CrossRef]
- Chang, H.; Olson, S.L. Examining Early Behavioral Persistence as a Dynamic Process: Correlates and Consequences Spanning Ages 3–10 Years. J. Abnorm. Child Psychol. 2016, 44, 799–810. [Google Scholar] [CrossRef]
- Pleeging, E.; Burger, M.; van Exel, J. The Relations between Hope and Subjective Well-Being: A Literature Overview and Empirical Analysis. Appl. Res. Qual. Life 2021, 16, 1019–1041. [Google Scholar] [CrossRef]
- Basieva, I.; Cervantes, V.H.; Dzhafarov, E.N.; Khrennikov, A. True contextuality beats direct influences in human decision making. J. Exp. Psychol. Gen. 2019, 148, 1925–1937. [Google Scholar] [CrossRef]
- Chang, L.W.; Gershman, S.J.; Cikara, M. Comparing value coding models of context-dependence in social choice. J. Exp. Soc. Psychol. 2019, 85, 103847. [Google Scholar] [CrossRef]
- Friston, K.J.; Wiese, W.; Hobson, J.A. Sentience and the Origins of Consciousness: From Cartesian Duality to Markovian Monism. Entropy 2020, 22, 516. [Google Scholar] [CrossRef]
- Piscopo, D.M.; Weible, A.P.; Rothbart, M.K.; Posner, M.I.; Niell, C.M. Changes in white matter in mice resulting from low-frequency brain stimulation. Proc. Natl. Acad. Sci. USA 2018, 115, E6339–E6346. [Google Scholar] [CrossRef]
- Tomasi, D.G.; Shokri-Kojori, E.; Volkow, N.D. Brain Network Dynamics Adhere to a Power Law. Front. Neurosci. 2017, 11, 72. [Google Scholar] [CrossRef]
- Tozzi, A.; Peters, J.F. From Abstract Topology to Real Thermodynamic Brain activity. Cogn Neurodyn. 2017, 11, 283–292. [Google Scholar] [CrossRef]
- Prentner, R. Consciousness and Topologically Structured Phenomenal Spaces. Conscious. Cogn. 2019, 70, 25–38. [Google Scholar] [CrossRef]
- Libet, B.; Gleason, C.A.; Wright, E.W.; Pearl, D.K. Time of Conscious Intention to Act in Relation to Onset of Cerebral Activity (Readiness-Potential). The Unconscious Initiation of a Freely Voluntary Act. Brain 1983, 106, 623–642. [Google Scholar] [CrossRef]
- Landé, A. Unity in quantum theory. Found. Phys. 1971, 1, 191–202. [Google Scholar] [CrossRef]
- Lubashevsky, I. Psychophysical Laws as Reflection of Mental Space Properties. Phys. Life Rev. 2019, 31, 276–303. [Google Scholar] [CrossRef]
- Selesnick, S.A.; Piccinini, G. Quantum-Like Behavior without Quantum Physics II. A Quantum-Like Model of Neural Network Dynamics. J. Biol. Phys. 2018, 44, 501–538. [Google Scholar] [CrossRef] [PubMed]
- Luck, S.J.; Gaspelin, N.; Folk, C.L.; Remington, R.W.; Theeuwes, J. Progress toward resolving the attentional capture debate. Vis. Cogn. 2021, 29, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Busemeyer, J.R.; Wang, Z. What is quantum cognition, and how is it applied to psychology? Curr. Dir. Psychol. Sci. 2015, 24, 163–169. [Google Scholar] [CrossRef]
- Fioretti, M.; Vostroknutov, A.; Coricelli, G. Dynamic Regret Avoidance. Am. Econ. J. Microecon. 2022, 14, 70–93. [Google Scholar] [CrossRef]
- Pariser, E. The Filter Bubble: What the Internet Is Hiding from You; Penguin Books Limited: London, UK, 2011. [Google Scholar]
- Alodjants, A.P.; Bazhenov, A.Y.; Khrennikov, A.Y.; Bukhanovsky, A.V. Mean-field theory of social laser. Sci. Rep. 2022, 12, 8566. [Google Scholar] [CrossRef] [PubMed]
- Panksepp, J. Behavior. Empathy and the laws of affect. Science 2011, 334, 1358–1359. [Google Scholar] [CrossRef]
- Smith, M.L.; Asada, N.; Malenka, R.C. Anterior Cingulate Inputs to Nucleus Accumbens Control the Social Transfer of Pain and Analgesia. Science 2021, 371, 153–159. [Google Scholar] [CrossRef]
- Zeng, R.; Zhu, D. A Model and Simulation of the Emotional Contagion of Netizens in the Process of Rumor Refutation. Sci. Rep. 2019, 9, 14164. [Google Scholar] [CrossRef]
- Greaves, H.; Thomas, T. Studies in History and Philosophy of Science Part B: Studies in History and Philosophy of Modern Physics. Available online: https://www.sciencedirect.com/journal/studies-in-history-and-philosophy-of-science-part-b-studies-in-history-and-philosophy-of-modern-physics (accessed on 14 November 2023).
- Guo, Y.; Liu, Z.; Tang, H. Experimental Demonstration of Input-Output Indefiniteness in a Single Quantum Device. Available online: https://arxiv.org/abs/2210.17046 (accessed on 14 November 2023).
- Viermann, C.; Sparn, M.; Liebster, N.; Hans, M.; Kath, E.; Parra-López, A.; Tolosa-Simeón, M.; Sánchez-Kuntz, N.; Haas, T.; Strobel, H.; et al. Quantum field simulator for dynamics in curved space-time. Nature 2022, 611, 260–264. [Google Scholar] [CrossRef]
- Born, M. Zur Quantenmechanik der Stossvorgange Quantum Mechanics of Collision in Zeitschrift fur Physik 37 pp. 863–867, 1926. Quantenmechanik der Stossvorgange in Zeitschrift fur Physik 38 pp. 803–827, 1926. Das Adiabatenprinzip in. Atticus Rare Books. Available online: https://www.atticusrarebooks.com/pages/books/596/max-born/zur-quantenmechanik-der-stossvorgange-quantum-mechanics-of-collision-in-zeitschrift-fur-physik (accessed on 8 November 2023).
- Don, A.P.H.; Peters, J.F.; Ramanna, S.; Tozzi, A. Topological View of Flows Inside the BOLD Spontaneous Activity of the Human Brain. Front. Comput. Neurosci. 2020, 14, 34. [Google Scholar]
- Kremps, J.A.; Hahnel-Peeters, R.K.; Merrie, L.A.; Williams, K.E.; Sznycer, D. Sometimes We Want Vicious Friends: People Have Nuanced Preferences for How They Want Their Friends to Behave Toward Them Versus Others. Evol. Hum. Behav. 2023, 44, 88–98. [Google Scholar] [CrossRef]
- Khrennikov, A. Social Laser Model for the Bandwagon Effect: Generation of Coherent Information Waves. Entropy 2020, 22, 559. [Google Scholar] [CrossRef] [PubMed]
- Khrennikov, A. Social Laser as a Tool for Social Engineering with Illustration by COVID19 Protests, Pro-War and Anti-War Beaming[v1]|Preprints.org. Available online: https://www.preprints.org/manuscript/202210.0343/v1 (accessed on 14 November 2023).
- Boxell, L.; Gentzkow, M.; Shapiro, J.M. Cross-Country Trends in Affective Polarization. Rev. Econ. Stat. 2022, 1–60. [Google Scholar] [CrossRef]
- McCoy, J.; Rahman, T.; Somer, M. Polarization and the global crisis of democracy: Common patterns, dynamics, and pernicious consequences for democratic polities. Am. Behav. Sci. 2018, 62, 16–42. [Google Scholar] [CrossRef]
- Kristoufek, L.; Vosvrda, M. Herding, minority game, market clearing and efficient markets in a simple spin model framework. Commun. Nonlinear Sci. Numer. Simul. 2018, 54, 148–155. [Google Scholar] [CrossRef]
- León-Medina, F.J. Endogenous Changes in Public Opinion Dynamics. J. Artif. Soc. Soc. Simul. 2019, 22, 4. [Google Scholar] [CrossRef]
- Zha, Q.; Kou, G.; Zhang, H.; Liang, H.; Chen, X.; Li, C.C.; Dong, Y. Opinion Dynamics in Finance and Business: A Literature Review and Research Opportunities. Financ. Innov. 2020, 6, 1–22. [Google Scholar] [CrossRef]
- Vazquez, F.; Saintier, N.; Pinasco, J.P. Role of Voting Intention in Public Opinion Polarization. Phys. Rev. E 2020, 101, 012101. [Google Scholar] [CrossRef] [PubMed]
- Salehi, S.; Taghiyareh, F. Introspective Agents in Opinion Formation Modeling to Predict Social Market. In Proceedings of the 2019 5th International Conference on Web Research (ICWR), Tehran, Iran, 24–25 April 2019. [Google Scholar]
- Yih, J.; Uusberg, A.; Taxer, J.L.; Gross, J.J. Better Together: A Unified Perspective on Appraisal and Emotion Regulation. Cogn. Emot. 2019, 33, 41–47. [Google Scholar] [CrossRef]
- Stavrova, O.; Ehlebracht, D. The cynical genius illusion: Exploring and debunking lay beliefs about cynicism and competence. Pers. Soc. Psychol. Bull. 2019, 45, 254–269. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, S.; Zheng, X.; Liu, W. The Role of Cynicism and Personal Traits in the Organizational Political Climate and Sustainable Creativity. Sustainability 2019, 11, 257. [Google Scholar] [CrossRef]
- Saaty, T.L.; Vargas, L.G. Origin of Neural Firing and Synthesis in Making Comparisons. Eur. J. Pure Appl. Math. 2017, 10, 602–613. [Google Scholar]
- Magee, J.C.; Smith, P.K. The Social Distance Theory of Power. Pers. Soc. Psychol. Rev. 2013, 17, 158–186. [Google Scholar] [CrossRef] [PubMed]
- Maglio, S.J.; Trope, Y.; Liberman, N. Distance from a distance: Psychological distance reduces sensitivity to any further psychological distance. J. Exp. Psychol. Gen. 2013, 142, 644–657. [Google Scholar] [CrossRef]
- Du, J.; An, Y.; Ding, X.; Zhang, Q.; Xu, W. State mindfulness and positive emotions in daily life: An upward spiral process. Personal. Individ. Differ. 2019, 141, 57–61. [Google Scholar] [CrossRef]
- Phan, H.P.; Ngu, B.H.; Lin, R.-Y.; Wang, H.-W.; Shih, J.-H.; Shi, S.-Y. Predicting and enhancing students’ positive emotions: An empirical study from a Taiwanese sociocultural context. Heliyon 2019, 5, e02550. [Google Scholar] [CrossRef] [PubMed]
- Sato, W.; Kochiyama, T.; Uono, S.; Sawada, R.; Yoshikawa, S. Amygdala activity related to perceived social support. Sci. Rep. 2020, 10, 2951. [Google Scholar] [CrossRef]
- Van Berkel, L.; Crandall, C.S.; Eidelman, S.; Blanchar, J.C. Hierarchy, Dominance, and Deliberation: Egalitarian Values Require Mental Effort. Pers. Soc. Psychol. Bull. 2015, 41, 1207–1222. [Google Scholar] [CrossRef] [PubMed]
- Peil, K.T. Emotion: The Self-regulatory Sense. Glob. Adv. Health Med. 2014, 3, 80–108. [Google Scholar] [CrossRef] [PubMed]
- Beall, A.T.; Tracy, J.L. Emotivational psychology: How distinct emotions facilitate fundamental motives. Soc. Personal. Psychol. Compass 2017, 11, e12303. [Google Scholar] [CrossRef]
- Troy, A.S.; Shallcross, A.J.; Brunner, A.; Friedman, R.; Jones, M.C. Cognitive reappraisal and acceptance: Effects on emotion, physiology, and perceived cognitive costs. Emotion 2018, 18, 58–74. [Google Scholar] [CrossRef]
- Roberts, B.W. Time Reversal. Available online: https://philsci-archive.pitt.edu/15033/ (accessed on 14 November 2023).
- Zanin, M.; Güntekin, B.; Aktürk, T. Time Irreversibility of Resting-State Activity in the Healthy Brain and Pathology. Front. Physiol. 2020, 10, 1619. [Google Scholar] [CrossRef]
- Sanacora, G.; Yan, Z.; Popoli, M. The Stressed Synapse 2.0: Pathophysiological Mechanisms in Stress-Related Neuropsychiatric Disorders. Nat. Rev. Neurosci. 2022, 23, 86–103. [Google Scholar] [CrossRef] [PubMed]
- Fayolle, S.; Gil, S.; Droit-Volet, S. Fear and time: Fear speeds up the internal clock. Behav. Processes 2015, 120, 135–140. [Google Scholar] [CrossRef]
- Esghaei, M.; Treue, S.; Vidyasagar, T. Dynamic Coupling of Oscillatory Neural Activity and Its Roles in Visual Attention. Trends Neurosci. 2022, 45, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Cui, X.; Wan, W.; Zheng, W.; Gu, Z. Long-range correlation analysis of high frequency prefrontal electroencephalogram oscillations for dynamic emotion recognition. Biomed. Signal Process. Control 2022, 72, 103291. [Google Scholar] [CrossRef]
- Baez-Lugo, S.; Deza-Araujo, Y.I.; Maradan, C.; Collette, F.; Lutz, A.; Marchant, N.L.; Vuilleumier, P.; Klimecki, O.; Arenaza-Urquijo, E.; André, C.; et al. Exposure to negative socio-emotional events induces sustained alteration of resting-state brain networks in older adults. Nat. Aging 2023, 3, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Laws, B. The return of the suppressed: Exploring how emotional suppression reappears as violence and pain among male and female prisoners. Punishm. Soc. 2019, 21, 560–577. [Google Scholar] [CrossRef]
- Ruan, Y.; Reis, H.T.; Zareba, W.; Lane, R.D. Does suppressing negative emotion impair subsequent emotions? Two experience sampling studies. Motiv. Emot. 2020, 44, 427–435. [Google Scholar] [CrossRef]
- Sedighimornani, N. Is Shame Managed Through Mind-Wandering? Eur. J. Psychol. 2019, 15, 717–732. [Google Scholar] [CrossRef]
- Dureux, A.; Zanini, A.; Everling, S. Perception of Dynamic Facial Expressions Activates a Cortico-Subcortico-Cerebellar Network in Marmosets. bioRxiv. 2022. Available online: https://www.biorxiv.org/content/10.1101/2022.08.02.502528v1.full (accessed on 14 November 2023).
- Apazoglou, K.; Küng, A.-L.; Cordera, P.; Aubry, J.-M.; Dayer, A.; Vuilleumier, P.; Piguet, C. Rumination related activity in brain networks mediating attentional switching in euthymic bipolar patients. Int. J. Bipolar Disord. 2019, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Flechsenhar, A.; Levine, S.; Bertsch, K. Threat induction biases processing of emotional expressions. Front. Psychol. 2022, 13, 967800. [Google Scholar] [CrossRef]
- Wang, Z. Brain Entropy Mapping in Healthy Aging and Alzheimer’s Disease-PMC. Front Aging Neurosci. 2020, 12, 596122. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Ye, L.; Yang, Q.; Cui, Q.; He, Z.; Li, L.; Yang, X.; Zou, Q.; Yang, P.; et al. Spatial complexity of brain signal is altered in patients with generalized anxiety disorder. J. Affect. Disord. 2019, 246, 387–393. [Google Scholar] [CrossRef]
- Saarimäki, H.; Ejtehadian, L.F.; Glerean, E.; Jääskeläinen, I.P.; Vuilleumier, P.; Sams, M.; Nummenmaa, L. Distributed affective space represents multiple emotion categories across the human brain. Soc. Cogn. Affect. Neurosci. 2018, 13, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Wiehler, A.; Branzoli, F.; Adanyeguh, I.; Mochel, F.; Pessiglione, M. A neuro-metabolic account of why daylong cognitive work alters the control of economic decisions. Curr. Biol. 2022, 32, 3564–3575.e5. [Google Scholar] [CrossRef]
- Aberg, K.C.; Toren, I.; Paz, R. Irrelevant Threats Linger and Affect Behavior in High Anxiety. J. Neurosci. 2023, 43, 656–671. [Google Scholar] [CrossRef]
- Nuno-Perez, A.; Trusel, M.; Lalive, A.L.; Congiu, M.; Gastaldo, D.; Tchenio, A.; Lecca, S.; Soiza-Reilly, M.; Bagni, C.; Mameli, M. Stress Undermines Reward-Guided Cognitive Performance through Synaptic Depression in the Lateral Habenula. Neuron 2021, 109, 947–956. [Google Scholar] [CrossRef]
- Alhussien, M.N.; Dang, A.K. Interaction between stress hormones and phagocytic cells and its effect on the health status of dairy cows: A review. Vet. World 2020, 13, 1837–1848. [Google Scholar] [CrossRef]
- Kekic, M.; McClelland, J.; Bartholdy, S.; Chamali, R.; Campbell, I.C.; Schmidt, U. Bad things come to those who do not wait: Temporal discounting is associated with compulsive overeating, eating disorder psychopathology and food addiction. Front. Psychiatry 2020, 10, 978. [Google Scholar] [CrossRef]
- Koomen, R.; Grueneisen, S.; Herrmann, E. Children Delay Gratification for Cooperative Ends. Psychol. Sci. 2020, 31, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Contreras, A.; Valiente, C.; Heeren, A.; Bentall, R. A temporal network approach to paranoia: A pilot study. Front. Psychol. 2020, 11. [Google Scholar] [CrossRef]
- Greene, T.; Gelkopf, M.; Fried, F.; Robinaugh, D.J.; Pickman, L.L. Dynamic Network Analysis of Negative Emotions and DSM-5 Posttraumatic Stress Disorder Symptom Clusters During Conflict. J. Trauma Stress. 2020, 33, 72–83. [Google Scholar] [CrossRef]
- Picard, M.; McEwen, B.S.; Epel, E.S.; Sandi, C. An energetic view of stress: Focus on mitochondria. Front. Neuroendocrinol. 2018, 49, 72–85. [Google Scholar] [CrossRef]
- Trevisiol, A.; Saab, A.S.; Winkler, U.; Marx, G.; Imamura, H.; Möbius, W.; Kusch, K.; Nave, K.-A.; Hirrlinger, J. Monitoring ATP dynamics in electrically active white matter tracts. eLife 2017, 6, e24241. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, Z.; Li, L.; Zhou, Y.; Lin, D.; Zhang, M.; Zhang, L.; Huang, G.; Liu, X.; Becker, B.; et al. Functional connectivity profiles of the default mode and visual networks reflect temporal accumulative effects of sustained naturalistic emotional experience. NeuroImage 2023, 269, 119941. [Google Scholar] [CrossRef]
- Sollenberger, N.A.; Kimbler, A.; Cummings, L.R.; Pettit, J.W.; Hayes, T.; Patriarca, G.C.; Vázquez, A.L.; Shumway, P.; Yegüez, C.E.; Rey, Y.; et al. Sleep fails to depotentiate amygdala-reactivity to negative emotional stimuli in youth with elevated symptoms of anxiety. Cogn. Affect. Behav. Neurosci. 2023, 23, 415–426. [Google Scholar] [CrossRef]
- Huang, J.P.Y.; Trull, T.J.; Merrill, A.M.; Myers, O.T.; Straub, K.T.; Kerns, J.G. Daily-Life Negative Affect in Emotional Distress Disorders Associated with Altered Frontoinsular Emotion Regulation Activation and Cortical Gyrification. Cogn. Ther. Res. 2021, 45, 1–18. [Google Scholar]
- Sugimura, K.; Iwasa, Y.; Kobayashi, R.; Honda, T.; Hashimoto, J.; Kashihara, S.; Zhu, J.; Yamamoto, K.; Kawahara, T.; Anno, M.; et al. Association between Long-Range Temporal Correlations in Intrinsic EEG Activity and Subjective Sense of Identity. Sci. Rep. 2021, 11, 422. [Google Scholar] [CrossRef] [PubMed]
- Saarimäki, H.; Gotsopoulos, A.; Jääskeläinen, I.P.; Lampinen, J.; Vuilleumier, P.; Hari, R.; Sams, M.; Nummenmaa, L. Discrete neural signatures of basic emotions. Cereb. Cortex 2016, 26, 2563–2573. [Google Scholar] [CrossRef] [PubMed]
- Kooij, D.T.A.M.; Kanfer, R.; Betts, M.; Rudolph, C.W. Future time perspective: A systematic review and meta-analysis. J. Appl. Psychol. 2018, 103, 867–893. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dai, Y.; Hao, L.; Song, L. Social Media and Attitude Change: Information Booming Promote or Resist Persuasion? Front. Psychol. 2021, 12, 596071. [Google Scholar] [CrossRef] [PubMed]
- Haimovitz, K.; Dweck, C.S.; Walton, G.M. Preschoolers find ways to resist temptation after learning that willpower can be energizing. Dev. Sci. 2020, 23, e12905. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, R.; Wu, J.; Yang, Q.; Zheng, S.; Wu, K. Psychological resilience, self-acceptance, perceived social support and their associations with mental health of incarcerated offenders in China. Asian J. Psychiatry 2020, 52, 102166. [Google Scholar] [CrossRef]
- Jans-Beken, L.; Jacobs, N.; Janssens, M.; Peeters, S.; Reijnders, J.; Lechner, L.; Lataster, J. Gratitude and health: An updated review. J. Posit. Psychol. 2020, 15, 743–782. [Google Scholar] [CrossRef]
- Ng, R.; Allore, H.G.; Levy, B.R. Self-Acceptance and Interdependence Promote Longevity: Evidence from a 20-Year Prospective Cohort Study. Int. J. Environ. Res. Public Health 2020, 17, 5980. [Google Scholar] [CrossRef] [PubMed]
- Keshmiri, S. Entropy and the Brain: An Overview. Entropy. 2020, 22, 917. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, Z.; Cui, H.; Zhang, T.; Zhao, L.; He, Z.; Liu, H.; Guo, L.; Liu, T.; Becker, B.; et al. Temporal Variability of Cortical Gyral-Sulcal Resting State Functional Activity Correlates With Fluid Intelligence. Front. Neural Circuits 2019, 13, 36. [Google Scholar] [CrossRef]
- Zmigrod, L.; Zmigrod, S.; Rentfrow, P.J.; Robbins, T.W. The psychological roots of intellectual humility: The role of intelligence and cognitive flexibility. Personal. Individ. Differ. 2019, 141, 200–208. [Google Scholar] [CrossRef]
- Shi, L.; Beaty, R.E.; Chen, Q.; Sun, J.; Wei, D.; Yang, W.; Qiu, J. Brain Entropy is Associated with Divergent Thinking. Cereb. Cortex 2020, 30, 708–717. [Google Scholar] [CrossRef]
- Brockman, R.; Ciarrochi, J.; Parker, P.; Kashdan, T. Emotion regulation strategies in daily life: Mindfulness, cognitive reappraisal and emotion suppression. Cogn. Behav. Ther. 2017, 46, 91–113. [Google Scholar] [CrossRef] [PubMed]
- Cockburn, J.; Man, V.; Cunningham, W.A.; O’doherty, J.P. Novelty and uncertainty regulate the balance between exploration and exploitation through distinct mechanisms in the human brain. Neuron 2022, 110, 2691–2702.e8. [Google Scholar] [CrossRef] [PubMed]
- Carmona–Halty, M.; Salanova, M.; Llorens, S.; Schaufeli, W.B. How Psychological Capital Mediates Between Study–Related Positive Emotions and Academic Performance. J. Happiness Stud. 2019, 20, 605–617. [Google Scholar] [CrossRef]
- Gutiérrez-Zamora Velasco, G.; Fernández, T.; Silva-Pereyra, J.; Reynoso-Alcántara, V.; Castro-Chavira, S.A. Higher Cognitive Reserve Is Associated with Better Working Memory Performance and Working-Memory-Related P300 Modulation. Brain Sci. 2021, 11, 308. [Google Scholar] [CrossRef] [PubMed]
- Bongers, A.; Flynn, A.B.; Northoff, G. Is learning scale-free? Chemistry learning increases EEG fractal power and changes the power law exponent. Neurosci. Res. 2020, 156, 165–177. [Google Scholar] [CrossRef]
- Debatin, T. A Revised Mental Energy Hypothesis of the g Factor in Light of Recent Neuroscience. Rev. Gen. Psychol. 2019, 23, 201–210. [Google Scholar] [CrossRef]
- Dupree, E.J.; Goodwin, A.; Darie, C.C.; Boolani, A. A Pilot Exploratory Proteomics Investigation of Mental Fatigue and Mental Energy. Adv. Exp. Med. Biol. 2019, 1140, 601–611. [Google Scholar] [CrossRef]
- Deak, A.; Bodrogi, B.; Orsi, G.; Perlaki, G.; Bereczkei, T. Emotional Intelligence Not Only Can Make Us Feel Negative, but Can Provide Cognitive Resources to Regulate It Effectively: An fMRI Study. Front. Psychol. 2022, 13, 866933. [Google Scholar] [CrossRef]



| Particle Type | Fermions | Consciousness |
|---|---|---|
| Quantum state | Wave function | Quantum cognition |
| Quantum mechanics | Individual particle behavior | Psychology and social sciences |
| Spin | An intrinsic angular momentum | The thermodynamic cycle’s direction |
| Pauli exclusion principle | Fermions cannot simultaneously occupy the same quantum state | A need for personal space and territorial needs |
| Complementarity | The context generated by the first measurement influences the next one | The context of the first question modulates subsequent responses |
| Wave-particle duality | The wave function collapses | Chaotic and probabilistic thoughts resolve into unified decisions |
| The Thermodynamic Cycle of Cognition | Endothermic: Reversed Carnot Cycle | Exothermic: Carnot Cycle |
|---|---|---|
| Entropy | High entropy resting state | Low entropy resting state |
| Mental state | Positive emotions, novelty | Negative emotions, repetitious thinking, aggravation, and violence |
| Temporal orientation | Future orientation | Past focus |
| Degrees of freedom | Expanding degrees of freedom | Loss of degrees of freedom |
| Thermodynamic consequences | An endothermic cycle absorbs energy and entropy from the environment. | An exothermic cycle dumps energy and entropy onto the environment. |
| Consequences for the organism | Mental energy accumulation (intellect) | Mental energy degradation → insecurity, mental and immune problems, depression |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deli, E.K. What Is Psychological Spin? A Thermodynamic Framework for Emotions and Social Behavior. Psych 2023, 5, 1224-1240. https://doi.org/10.3390/psych5040081
Deli EK. What Is Psychological Spin? A Thermodynamic Framework for Emotions and Social Behavior. Psych. 2023; 5(4):1224-1240. https://doi.org/10.3390/psych5040081
Chicago/Turabian StyleDeli, Eva K. 2023. "What Is Psychological Spin? A Thermodynamic Framework for Emotions and Social Behavior" Psych 5, no. 4: 1224-1240. https://doi.org/10.3390/psych5040081