Conversion Study on the Formation of Mechanochemically Synthesized BaTiO3
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Ball-Milling Experiments
2.2. Characterization
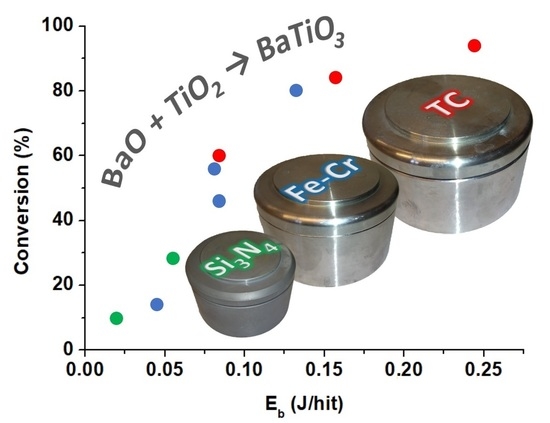
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vijatovic, M.; Bobic, J.; Stojanovic, B. History and challenges of barium titanate: Part I. Sci. Sinter. 2008, 40, 155–165. [Google Scholar] [CrossRef]
- Jiang, B.; Iocozzia, J.; Zhao, L.; Zhang, H.; Harn, Y.-W.; Chen, Y.; Lin, Z. Barium titanate at the nanoscale: Controlled synthesis and dielectric and ferroelectric properties. Chem. Soc. Rev. 2019, 48, 1194–1228. [Google Scholar] [CrossRef] [PubMed]
- Cinčić, D.; Brekalo, I.; Kaitner, B. Effect of atmosphere on solid-state amine–aldehyde condensations: Gas-phase catalysts for solid-state transformations. Chem. Commun. 2012, 48, 11683–11685. [Google Scholar] [CrossRef]
- Stolar, T.; Užarević, K. Mechanochemistry: An efficient and versatile toolbox for synthesis, transformation, and functionalization of porous metal–organic frameworks. CrystEngComm 2020, 22, 4511–4525. [Google Scholar] [CrossRef]
- Tan, D.; Loots, L.; Friščić, T. Towards medicinal mechanochemistry: Evolution of milling from pharmaceutical solid form screening to the synthesis of active pharmaceutical ingredients (APIs). Chem. Commun. 2016, 52, 7760–7781. [Google Scholar] [CrossRef] [PubMed]
- Stojanović, B.D.; Jovalekić, C.; Vukotic, V.; Simoes, A.Z.; Varela, J.A. Ferroelectric Properties of Mechanically Synthesized Nanosized Barium Titanate. Ferroelectrics 2005, 319, 65–73. [Google Scholar] [CrossRef]
- Stojanovic, B.; Simoes, A.; Paiva-Santos, C.; Jovalekic, C.; Mitic, V.; Varela, J. Mechanochemical synthesis of barium titanate. J. Eur. Ceram. Soc. 2005, 25, 1985–1989. [Google Scholar] [CrossRef]
- Garbarz-Glos, B.; Bak, W.; Budziak, A.; Dulian, P.; Lisinka-Czekaj, A.; Czekaj, D. The application of the mechanochemical synthesis for the preparation of advanced ceramics based on barium titanate. Arch. Metall. Mater. 2020, 65, 1391–1396. [Google Scholar]
- van Hal, H.; Groen, W.; Maassen, S.; Keur, W. Mechanochemical synthesis of BaTiO3, Bi0.5Na0.5TiO3 and Ba2NaNb5O15 dielectric ceramics. J. Eur. Ceram. Soc. 2001, 21, 1689–1692. [Google Scholar] [CrossRef]
- Aydin, Z.; Turgut, S.; Akbas, H.Z. Structural Differences of BaTiO3 Ceramics Modified by Ultrasonic and Mechanochemical Methods. Sov. Powder Met. Met. Ceram. 2018, 57, 490–497. [Google Scholar] [CrossRef]
- Jost, W. Fast Reactions in Solids, von FP Bowden und AD Yoffe; Butterworths Scientific Publications: London, UK, 1958; Volume 71, p. 752. [Google Scholar]
- Weichert, R.; Schönert, K. On the temperature rise at the tip of a fast running crack. J. Mech. Phys. Solids 1974, 22, 127–133. [Google Scholar] [CrossRef]
- Gomes, M.A.; Lima, A.; Eguiluz, K.; Salazar-Banda, G.R. Wet chemical synthesis of rare earth-doped barium titanate nanoparticles. J. Mater. Sci. 2016, 51, 4709–4727. [Google Scholar] [CrossRef]
- Seo, K.W.; Kong, H.G. Hydrothermal preparation of BaTiO3 thin films. Korean J. Chem. Eng. 2000, 17, 428–432. [Google Scholar] [CrossRef]
- Sun, W.; Li, C.; Li, J.; Liu, W. Microwave-hydrothermal synthesis of tetragonal BaTiO3 under various conditions. Mater. Chem. Phys. 2006, 97, 481–487. [Google Scholar] [CrossRef]
- Catauro, M.; Tranquillo, E.; Poggetto, G.D.; Naviglio, S.; Barrino, F. Antibacterial Properties of Sol–Gel Biomaterials with Different Percentages of PEG or PCL. Macromol. Symp. 2020, 389, 1900056. [Google Scholar] [CrossRef]
- Šagud, I.; Zanolla, D.; Perissutti, B.; Passerini, N.; Škorić, I. Identification of degradation products of praziquantel during the mechanochemical activation. J. Pharm. Biomed. Anal. 2018, 159, 291–295. [Google Scholar] [CrossRef]
- Burgio, N.; Iasonna, A.; Magini, M.; Martelli, S.; Padella, F. Mechanical Alloying of the Fe-Zr System—Correlation between Input Energy and End-Products. Il Nuovo Cimento D 1991, 13, 459–476. [Google Scholar] [CrossRef]
- Kozma, G.; Puskás, R.; Papp, I.; Bélteky, P.; Kónya, Z.; Kukovecz, Á. Experimental validation of the Burgio–Rojac model of planetary ball milling by the length control of multiwall carbon nanotubes. Carbon 2016, 105, 615–621. [Google Scholar] [CrossRef]
- Andonova, S.M.; Senturk, G.S.; Kayhan, E.; Ozensoy, E. Nature of the Ti-Ba Interactions on the BaO/TiO2/Al2O3 NOx Storage System. J. Phys. Chem. C 2009, 113, 11014–11026. [Google Scholar] [CrossRef]
- Zhang, W.F.; He, Y.L.; Zhang, M.S.; Yin, Z.; Chen, Q. Raman scattering study on anatase TiO2 nanocrystals. J. Phys. D-Appl. Phys. 2000, 33, 912–916. [Google Scholar] [CrossRef]
- Venkateswaran, U.D.; Naik, V.M.; Naik, R. High-pressure Raman studies of polycrystalline BaTiO3. Phys. Rev. B 1998, 58, 14256–14260. [Google Scholar] [CrossRef]
- Shiratori, Y.; Pithan, C.; Dornseiffer, J.; Waser, R. Raman scattering studies on nanocrystalline BaTiO3 Part I—Isolated particles and aggregates. J. Raman Spectrosc. 2007, 38, 1288–1299. [Google Scholar] [CrossRef]
- Hayashi, H.; Nakamura, T.; Ebina, T. In-situ Raman spectroscopy of BaTiO3 particles for tetragonal–cubic transformation. J. Phys. Chem. Solids 2013, 74, 957–962. [Google Scholar] [CrossRef]
- Lazarevic, Z.; Romčević, N.; Vijatović, M.; Paunović, N.; Romčević, M.; Stojanović, B.; Dohčević-Mitrović, Z. Characterization of Barium Titanate Ceramic Powders by Raman Spectroscopy. Acta Phys. Pol. A 2009, 115, 808–810. [Google Scholar] [CrossRef]
- Sydorchuk, V.; Zazhigalov, V.A.; Khalameida, S.; Wieczorek-Ciurowa, K. Mechanochemical synthesis of BaTiO3 using different forms of TiO2. Inorg. Mater. 2010, 46, 1126–1130. [Google Scholar] [CrossRef]
- Siddheswaran, R.; Šutta, P.; Novák, P.; Netrvalová, M.; Hendrych, A.; Životský, O. In-situ X-ray diffraction studies and magneto-optic Kerr effect on RF sputtered thin films of BaTiO3 and Co, Nb co-doped BaTiO3. Ceram. Int. 2016, 42, 3882–3887. [Google Scholar] [CrossRef]
- Sun, Q.; Gu, Q.; Zhu, K.; Jin, R.; Liu, J.; Wang, J.; Qiu, J. Crystalline Structure, Defect Chemistry and Room Temperature Colossal Permittivity of Nd-doped Barium Titanate. Sci. Rep. 2017, 7, 42274. [Google Scholar] [CrossRef] [Green Version]
- Rojac, T.; Kosec, M.; Malič, B.; Holc, J. The application of a milling map in the mechanochemical synthesis of ceramic oxides. J. Eur. Ceram. Soc. 2006, 26, 3711–3716. [Google Scholar] [CrossRef]
- Magini, M.; Iasonna, A.; Padella, F. Ball milling: An experimental support to the energy transfer evaluated by the collision model. Scr. Mater. 1996, 34, 13–19. [Google Scholar] [CrossRef]
- Abdellaoui, M.; Gaffet, E. The physics of mechanical alloying in a planetary ball mill: Mathematical treatment. Acta Met. Mater. 1995, 43, 1087–1098. [Google Scholar] [CrossRef]
- Raja, S.; Bheeman, D.; Rajamani, R.; Pattiyappan, S.; Sugamaran, S.; Bellan, C.S. Synthesis, Characterization and Remedial Aspect of BaTiO3 Nanoparticles Against Bacteria. Nanomed. Nanobiol. 2015, 2, 16–20. [Google Scholar] [CrossRef]
- Rosenkranz, S.; Breitung-Faes, S.; Kwade, A. Experimental investigations and modelling of the ball motion in planetary ball mills. Powder Technol. 2011, 212, 224–230. [Google Scholar] [CrossRef]






| Grinding Vessel | ωp (1/s) | Nb (pcs) | Eb (J/hit) | Ecum (J/g) | Short Name |
|---|---|---|---|---|---|
| Si3N4 | 300 | 25 | 0.0197 | 5907 | SN300/25 |
| 500 | 25 | 0.0548 | 27,350 | SN500/25 | |
| FeNiCr | 300 | 25 | 0.0455 | 13,613 | FN300/25 |
| 400 | 25 | 0.0809 | 32,269 | FN400/25 | |
| 400 | 15 | 0.0835 | 19,989 | FN400/15 | |
| 500 | 10 | 0.132 | 26,311 | FN500/10 | |
| TC | 300 | 25 | 0.0845 | 25,282 | TC300/25 |
| 400 | 10 | 0.157 | 25,018 | TC400/10 | |
| 500 | 10 | 0.245 | 48,865 | TC500/10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozma, G.; Lipták, K.; Deák, C.; Rónavári, A.; Kukovecz, Á.; Kónya, Z. Conversion Study on the Formation of Mechanochemically Synthesized BaTiO3. Chemistry 2022, 4, 592-602. https://doi.org/10.3390/chemistry4020042
Kozma G, Lipták K, Deák C, Rónavári A, Kukovecz Á, Kónya Z. Conversion Study on the Formation of Mechanochemically Synthesized BaTiO3. Chemistry. 2022; 4(2):592-602. https://doi.org/10.3390/chemistry4020042
Chicago/Turabian StyleKozma, Gábor, Kata Lipták, Cora Deák, Andrea Rónavári, Ákos Kukovecz, and Zoltán Kónya. 2022. "Conversion Study on the Formation of Mechanochemically Synthesized BaTiO3" Chemistry 4, no. 2: 592-602. https://doi.org/10.3390/chemistry4020042