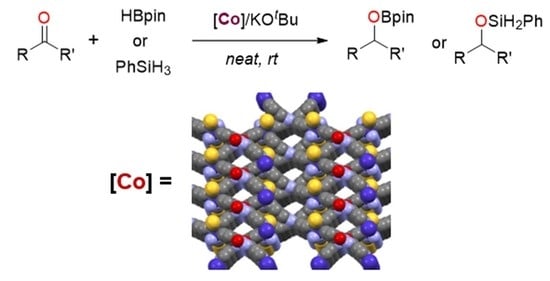
Assembly of a 3D Cobalt(II) Supramolecular Framework and Its Applications in Hydrofunctionalization of Ketones and Aldehydes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of 1
2.3. General Procedure for 1-Catalyzed Hydroboration of Ketones and Aldehydes
2.4. General Procedure for 1-Catalyzed Hydrosilylation of Ketones and Aldehydes
2.5. General Procedure for 1-Catalyzed Chemoselective Hydroboration
2.6. X-ray Crystallography
3. Results
3.1. Synthesis and Structural Characterization
3.2. Catalytic Applications
3.3. Chemoselectivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gangu, K.K.; Maddila, S.; Mukkamala, S.B.; Jonnalagadda, S.B. A Review on Contemporary Metal-Organic Framework Materials. Inorg. Chim. Acta 2016, 446, 61–74. [Google Scholar] [CrossRef]
- Cai, G.; Yan, P.; Zhang, L.; Zhou, H.C.; Jiang, H.L. Metal–organic framework-based hierarchically porous materials: Synthesis and applications. Chem. Rev. 2021, 121, 12278–12326. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, G.; Park, I.H.; Medishetty, R.; Vittal, J.J. Two-dimensional metal-organic framework materials: Synthesis, structures, properties and applications. Chem. Rev. 2021, 121, 3751–3891. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, S.; Uemura, K. Dynamic porous properties of coordination polymers inspired by hydrogen bonds. Chem. Soc. Rev. 2005, 34, 109–119. [Google Scholar] [CrossRef]
- MacDonald, J.C.; Dorrestein, P.C.; Pilley, M.M.; Foote, M.M.; Lundburg, J.L.; Henning, R.W.; Schultz, A.J.; Manson, J.L. Design of Layered Crystalline Materials Using Coordination Chemistry and Hydrogen Bonds. J. Am. Chem. Soc. 2000, 122, 11692–11702. [Google Scholar] [CrossRef]
- Cui, Y.; Ngo, H.L.; White, P.S.; Lin, W. Hierarchical Assembly of Homochiral Porous Solids Using Coordination and Hydrogen Bonds. Inorg. Chem. 2003, 42, 652–654. [Google Scholar] [CrossRef]
- Wu, X.; Wang, J.; Huang, J.; Yang, S. Robust, Stretchable, and Self-Healable Supramolecular Elastomers Synergistically Cross-Linked by Hydrogen Bonds and Coordination Bonds. ACS Appl. Mater. Interf. 2019, 11, 7387–7396. [Google Scholar] [CrossRef]
- Kwon, H.; Lee, E. Coordination preference of hexa(2-pyridyl)benzene with copper(II) directed by hydrogen bonding. CrystEngComm 2018, 20, 5233–5240. [Google Scholar] [CrossRef]
- Chang, C.C.; Kinjo, R. Catalytic Hydroboration of Carbonyl Derivatives, Imines, and Carbon Dioxide. ACS Catal. 2015, 5, 3238–3259. [Google Scholar] [CrossRef]
- Cho, B.T. Recent development and improvement for boron hydride-based catalytic asymmetric reduction of unsymmetrical ketones. Chem. Soc. Rev. 2009, 38, 443–452. [Google Scholar] [CrossRef]
- Wang, W.; Lu, K.; Qin, Y.; Yao, W.; Yuan, D.; Pullarkat, S.A.; Xu, L.; Ma, M. Grignard reagents-catalyzed hydroboration of aldehydes and ketones. Tetrahedron 2020, 76, 131145. [Google Scholar] [CrossRef]
- Tamang, S.R.; Findlater, M. Emergence and Applications of Base Metals (Fe, Co, and Ni) in Hydroboration and Hydrosilylation. Molecules 2019, 24, 3194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Park, S. Recent Advances in Metal-Catalyzed Asymmetric Hydroboration of Ketones. ChemCatChem 2021, 13, 1898–1919. [Google Scholar] [CrossRef]
- Shegavi, M.L.; Bose, S.K. Recent advances in the catalytic hydroboration of carbonyl compounds. Catal. Sci. Tech. 2019, 9, 3307–3336. [Google Scholar] [CrossRef]
- Jakhar, V.K.; Barman, M.K.; Nembenna, S. Aluminum monohydride catalyzed selective hydroboration of carbonyl compounds. Org. Lett. 2016, 18, 4710–4713. [Google Scholar] [CrossRef] [PubMed]
- Oluyadi, A.A.; Ma, S.; Muhoro, C.N. Titanocene(II)-Catalyzed Hydroboration of Carbonyl Compounds. Organometallics 2013, 32, 70–78. [Google Scholar] [CrossRef]
- Baishya, A.; Baruah, S.; Geetharani, K. Efficient hydroboration of carbonyls by an iron(II) amide catalyst. Dalton Trans. 2018, 47, 9231–9236. [Google Scholar] [CrossRef]
- Khoo, S.; Cao, J.; Ng, F.; So, C.W. Synthesis of a Base-Stabilized Silicon(I)–Iron(II) Complex for Hydroboration of Carbonyl Compounds. Inorg. Chem. 2018, 57, 12452–12455. [Google Scholar] [CrossRef]
- Vijjamarri, S.; O’Denius, T.M.; Yao, B.; Kubátová, A.; Du, G. Highly Selective Hydroboration of Carbonyls by a Manganese Catalyst: Insight into the Reaction Mechanism. Organometalics 2020, 39, 3375–3383. [Google Scholar] [CrossRef]
- Li, M.; Liu, X.; Cui, D. Catalytic hydroboration of carbonyl derivatives by using phosphinimino amide ligated magnesium complexes. Dalton Trans. 2021, 50, 13037–13041. [Google Scholar] [CrossRef]
- Kim, J.H.; Jaladi, A.K.; Kim, H.T.; An, D.K. Lithium tert-Butoxide-catalyzed Hydroboration of Carbonyl Compounds. Bull. Korean Chem. Soc. 2019, 40, 971–975. [Google Scholar] [CrossRef]
- Zhang, G.; Zeng, H.; Wu, J.; Yin, Z.; Zheng, S.; Fettinger, J.C. Highly Selective Hydroboration of Alkenes, Ketones and Aldehydes Catalyzed by a Well-Defined Manganese Complex. Angew. Chem. Int. Ed. 2016, 55, 14369–14372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wu, J.; Zeng, H.; Neary, M.C.; Devany, M.; Zheng, S.; Dub, P.A. Dearomatization and Functionalization of Terpyridine Ligands Leading to Unprecedented Zwitterionic Meisenheimer Aluminum Complexes and Their Use in Catalytic Hydroboration. ACS Catal. 2019, 9, 874–884. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, J.; Zheng, S.; Neary, M.C.; Mao, J.; Flores, M.; Trovitch, R.J.; Dub, P.A. Redox-Noninnocent Ligand-Supported Vanadium Catalysts for the Chemoselective Reduction of C=X (X=O, N) Functionalities. J. Am. Chem. Soc. 2019, 141, 15230–15239. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.H.; Jaladi, A.K.; Lee, J.H.; Kim, T.S.; Shin, W.K.; Hwang, H.; An, D.K. Catalytic Hydroboration of Aldehydes, Ketones, and Alkenes Using Potassium Carbonate: A Small Key to Big Transformation. ACS Omega 2019, 4, 15893–15903. [Google Scholar] [CrossRef] [Green Version]
- Nowicki, M.; Kuciński, K.; Hreczycho, G.; Hoffmann, M. Catalytic and non-catalytic hydroboration of carbonyls: Quantum-chemical studies. Org. Biomol. Chem. 2021, 19, 3004–3015. [Google Scholar] [CrossRef]
- Bage, A.D.; Nicholson, K.; Hunt, T.A.; Langer, T.; Thomas, S.P. The Hidden Role of Boranes and Borohydrides in Hydroboration Catalysis. ACS Catal. 2020, 10, 13479–13486. [Google Scholar] [CrossRef]
- Wu, J.; Zeng, H.S.; Cheng, J.; Zheng, S.P.; Golen, J.A.; Manke, D.R.; Zhang, G. Cobalt(II) coordination polymer as a precatalyst for selective hydroboration of aldehydes, ketones, and imines. J. Org. Chem. 2018, 83, 9442–9448. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, J.; Li, S.; Cass, S.; Zheng, S. Markovnikov-Selective Hydroboration of Vinylarenes Catalyzed by a Cobalt (II) Coordination Polymer. Org. Lett. 2018, 20, 7893–7897. [Google Scholar] [CrossRef]
- Zhang, G.; Li, S.; Wu, J.; Zeng, H.; Mo, Z.; Davis, K.; Zheng, S. Highly efficient and selective hydroboration of terminal and internal alkynes catalysed by a cobalt(II) coordination polymer. Org. Chem. Front. 2019, 6, 3228–3233. [Google Scholar] [CrossRef]
- Zhang, G.; Cheng, J.; Davis, K.; Bonifacio, M.G.; Zajaczkowski, C. Practical and selective hydroboration of aldehydes and ketones in air catalysed by an iron(II) coordination polymer. Green Chem. 2019, 21, 1114–1121. [Google Scholar] [CrossRef]
- Liu, E.; Li, L.; Cheng, J.; Zhang, G. Synthesis and structural characterization of dinuclear Zinc(II) and Europium(III) complexes based on a bis-hydrazone ligand. J. Mol. Struct. 2019, 1188, 1–6. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.Z.; Yang, C.; Liu, E.; Golen, J.A.; Rheingold, A.L.; Zhang, G. Synthesis and structural characterization of zinc(II) and cobalt(II) complexes based on multidentate hydrazone ligands. J. Mol. Struct. 2016, 1110, 180–184. [Google Scholar] [CrossRef] [Green Version]
- Parmar, B.; Rachuri, Y.; Bisht, K.K.; Suresh, E. Mixed-Ligand LMOF Fluorosensors for Detection of Cr(VI) Oxyanions and Fe3+/Pd2+ Cations in Aqueous Media. Inorg. Chem. 2017, 56, 10939–10949. [Google Scholar] [CrossRef]
- Roztocki, K.; Jędrzejowski, D.; Hodorowicz, M.; Senkovska, I.; Kaskel, S.; Matoga, D. Effect of Linker Substituent on Layers Arrangement, Stability, and Sorption of Zn-Isophthalate/Acylhydrazone Frameworks. Cryst. Growth Des. 2018, 18, 488–497. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXTL, An Integrated System for Solving, Refining, and Displaying Crystal Structures from Diffraction Data; University of Göttingen: Göttingen, Germany, 1981. [Google Scholar]
- Sheldrick, G.M. Crystal Structure Refinement by SHELXL. Acta Cryst. 2015, 71, 3–8. [Google Scholar]
- Spek, A.L. PLATON, A Multipurpose Crystallographic Tool; Utrecht University: Utrecht, The Netherlands, 2005. [Google Scholar]
- Spek, A.L. Single-Crystal Structure Validation with the Program PLATON. J. Appl. Cryst. 2003, 36, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.L.; Fan, W.W.; Lu, Z.X.; Qin, Y.; Yang, S.X.; Li, Y.; Liu, Y.X.; Zheng, L.Y.; Cao, Q.E. Solvent-Driven Reversible Phase Transition of a Pillared Metal–Organic Framework. Chem. Eur. J. 2019, 25, 5787–5792. [Google Scholar] [CrossRef]
- Krūkle-Bērziņa, K.; Belyakov, S.; Mishnev, A.; Shubin, K. Stability and Phase Transitions of Nontoxic γ-Cyclodextrin-K+ Metal-Organic Framework in Various Solvents. Crystals 2020, 10, 37. [Google Scholar] [CrossRef] [Green Version]
- Kundu, T.; Wahiduzzaman, M.; Shah, B.B.; Maurin, G.; Zhao, D. Solvent-induced control over breathing behavior in flexible metal–organic frameworks for natural-gas delivery. Angew. Chem. Int. Ed. 2019, 58, 8073–8077. [Google Scholar] [CrossRef]
- Zeng, H.; Wu, J.; Li, S.; Hui, C.; Ta, A.; Cheng, S.-Y.; Zheng, S.; Zhang, G. Copper(II)-Catalyzed Selective Hydroboration of Ketones and Aldehydes. Org. Lett. 2019, 21, 401. [Google Scholar] [CrossRef] [PubMed]




 | ||||
|---|---|---|---|---|
| Entry | Catalyst | Activator | Solvent | Yield/% b |
| 1 | 1 | - | THF | 0 |
| 2 | none | KOtBu | THF | <5 |
| 3 | Co(NCS)2 | KOtBu | THF | 68 |
| 4 | 1 | KOtBu | THF | 99 |
| 5 | [Co(4-pytpy)Cl2]n | KOtBu | THF | 99 |
| 6 | 1 | KOtBu | Et2O | 95 |
| 7 | 1 | KOtBu | toluene | 80 |
| 8 | 1 | KOtBu | benzene | 86 |
| 9 | 1 | KOtBu | pentane | 95 |
| 10 | 1 | KOtBu | none | 99 |
| 11 | 1 | KOH | none | 58 |
| 12 | 1 | K2CO3 | none | 67 |
| 13 | 1 | NaBH4 | none | 85 |
| 14 | 1 | NaBF4 | none | 18 |
| 15 | 1 | LiNTf2 | none | 96 |
 | ||||
|---|---|---|---|---|
| Entry | Substrate | Hydride | Product | Yield (%) b |
| 1 |  | HBpin |  | 92 |
| 2 |  | HBpin |  | 94 |
| 3 |  | HBpin |  | 92 |
| 4 |  | HBpin |  | 95 |
| 5 |  | HBpin |  | 89 |
| 6 |  | HBpin |  | 90 |
| 7 |  | HBpin |  | 82 |
| 8 |  | HBpin |  | 88 |
| 9 |  | HBpin |  | 90 |
| 10 |  | HBpin |  | 86 |
| 11 |  | PhSiH3 |  | 92 |
| 12 |  | PhSiH3 |  | 90 |
| 13 |  | PhSiH3 |  | 84 |
| 14 |  | PhSiH3 |  | 85 |
| 15 |  | PhSiH3 |  | 90 |
| 16 |  | HBpin |  | 5 c |
| 17 |  | HBpin |  | 3 c |
| 18 |  | PhSiH3 |  | trace c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Wang, A.; Zeng, H.; Zheng, S.; Neary, M.C. Assembly of a 3D Cobalt(II) Supramolecular Framework and Its Applications in Hydrofunctionalization of Ketones and Aldehydes. Chemistry 2022, 4, 393-404. https://doi.org/10.3390/chemistry4020029
Zhang G, Wang A, Zeng H, Zheng S, Neary MC. Assembly of a 3D Cobalt(II) Supramolecular Framework and Its Applications in Hydrofunctionalization of Ketones and Aldehydes. Chemistry. 2022; 4(2):393-404. https://doi.org/10.3390/chemistry4020029
Chicago/Turabian StyleZhang, Guoqi, Alex Wang, Haisu Zeng, Shengping Zheng, and Michelle C. Neary. 2022. "Assembly of a 3D Cobalt(II) Supramolecular Framework and Its Applications in Hydrofunctionalization of Ketones and Aldehydes" Chemistry 4, no. 2: 393-404. https://doi.org/10.3390/chemistry4020029