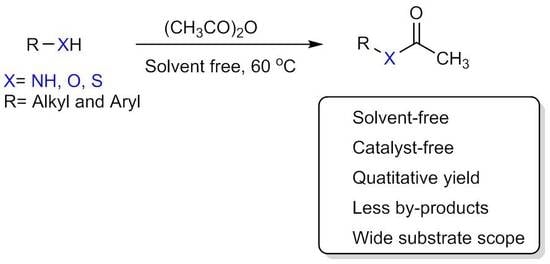
Acetylation of Alcohols, Amines, Phenols, Thiols under Catalyst and Solvent-Free Conditions
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. General Procedure for the Acetylation of Alcohols, Phenols, Thiols, and Amines under Solvent-Free Conditions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sartori, G.; Ballini, R.; Bigi, F.; Bosica, G.; Maggi, R.; Righi, P. Protection (and deprotection) of functional groups in organic synthesis by heterogeneous catalysis. Chem. Rev. 2004, 104, 199–250. [Google Scholar] [CrossRef] [PubMed]
- Greene, T.W.; Wuts, P.G.M. Protective Groups in Organic Synthesis, 3rd ed.; Wiley: New York, NY, USA, 1999. [Google Scholar]
- Iqbal, J.; Srivastava, R.R. Cobalt(II) chloride catalyzed acylation of alcohols with acetic anhydride: Scope and mechanism. J. Org. Chem. 1992, 57, 2001–2007. [Google Scholar] [CrossRef]
- Ishihara, K.; Kubota, M.; Kurihara, H.; Yamamoto, H. Scandium Trifluoromethane sulfonate as an Extremely Active Lewis Acid Catalyst in Acylation of Alcohols with Acid Anhydrides and Mixed Anhydrides. J. Org. Chem. 1996, 61, 4560–4567. [Google Scholar] [CrossRef] [PubMed]
- Li, A.-X.; Li, T.-S.; Ding, T.-H. Montmorillonite K-10 and KSF as remarkable acetylation catalysts. Chem. Commun. 1997, 15, 1389–1390. [Google Scholar] [CrossRef]
- Breton, G.W.; Kurtz, M.J.; Kurtz, S.L. Acetylation of Unsymmetrical Diols in the Presence of Al2O3. Tetrahedron Lett. 1997, 38, 3825–3828. [Google Scholar] [CrossRef]
- Ballini, R.; Bosica, G.; Carloni, S.; Ciaralli, L.; Maggi, R.; Sartori, G. Zeolite HSZ-360 as a New Reusable Catalyst for the Direct Acetylation of Alcohols and Phenols Under Solventless Conditions. Tetrahedron Lett. 1998, 39, 6049–6052. [Google Scholar] [CrossRef]
- Orita, A.; Tanahashi, C.; Kakuda, A.; Otera, J. Highly Efficient and Versatile Acylation of Alcohols with Bi(OTf)3 as Catalyst. Angew. Chem. Int. Ed. 2000, 39, 2877–2879. [Google Scholar] [CrossRef]
- Chavan, S.P.; Anand, R.; Pasupathy, K.; Rao, B.S. Catalytic acetylation of alcohols, phenols, thiols and amines with zeolite H-FER under solventless conditions. Green Chem. 2001, 3, 320–322. [Google Scholar] [CrossRef]
- Orita, A.; Tanahashi, C.; Kakuda, A.; Otera, J. Highly Powerful and Practical Acylation of Alcohols with Acid Anhydride Catalyzed by Bi(OTf)3. J. Org. Chem. 2001, 66, 8926–8934. [Google Scholar] [CrossRef]
- Chandra, K.L.; Saravanan, P.; Singh, R.K.; Singh, V.K. Lewis acid catalyzed acylation reactions: Scope and limitations. Tetrahedron 2002, 58, 1369–1374. [Google Scholar] [CrossRef]
- Chakraborti, A.K.; Gulhane, R. Perchloric acid adsorbed on silica gel as a new, highly efficient, and versatile catalyst for acetylation of phenols, thiols, alcohols, and amines. Chem. Commun. 2003, 15, 1896–1897. [Google Scholar] [CrossRef]
- Scriven, E.F.V. 4-Dialkylaminopyridines: Super acylation and alkylation catalysts. Chem. Soc. Rev. 1983, 12, 129–161. [Google Scholar] [CrossRef]
- Vedejs, E.; Bennett, N.S.; Conn, L.M.; Diver, S.T.; Gingras, M.; Lin, S.; Oliver, P.A.; Peterson, M.J. Tributylphosphine-catalyzed acylations of alcohols: Scope and related reactions. J. Org. Chem. 1993, 58, 7286–7288. [Google Scholar] [CrossRef]
- Vedejs, E.; Diver, S.T. Tributylphosphine: A remarkable acylation catalyst. J. Am. Chem. Soc. 1993, 115, 3358–3359. [Google Scholar] [CrossRef]
- Sano, T.; Ohashi, K.; Oriyama, T. Remarkably Fast Acylation of Alcohols with Benzoyl Chloride Promoted by TMEDA. Synthesis 1999, 7, 1141–1144. [Google Scholar] [CrossRef]
- Gholap, A.R.; Venkatesan, K.; Daniel, T.; Lahoti, R.J.; Srinivasan, K.V. Ultrasound promoted acetylation of alcohols in room temperature ionic liquid under ambient conditions. Green Chem. 2003, 5, 693–696. [Google Scholar] [CrossRef]
- De, S.K. Ruthenium (III) Chloride Catalyzed Acylation of Alcohols, Phenols, Thiols and Amines. Tetrahedron Lett. 2004, 45, 2919–2922. [Google Scholar] [CrossRef]
- Torregiani, E.; Seu, G.; Minassi, A.; Appendino, G. Cerium (III) Chloride-Promoted Chemoselective Esterification of Phenolic Alcohols. Tetrahedron Lett. 2005, 46, 2193–2196. [Google Scholar] [CrossRef]
- Chakraborti, A.K.; Gulhane, R. Zirconium(IV) Chloride as a New, Highly Efficient, and Reusable Catalyst for Acetylation of Phenols, Thiols, Amines, and Alcohols under Solvent-Free Conditions. Synlett 2004, 627–630. [Google Scholar] [CrossRef]
- Reddy, T.S.; Narasimhulu, M.; Suryakiran, N.; Mahesh, K.C.; Ashalatha, K.; Venkateswarlu, Y. A Mild and Efficient Acetylation of Alcohols, Phenols and Amines with Acetic Anhydride Using La(NO3)3·6H2O as a Catalyst under Solvent-Free Conditions. Tetrahedron Lett. 2006, 47, 6825–6829. [Google Scholar] [CrossRef]
- Kamal, A.; Khan, M.N.A.; Reddy, K.S.; Srikanth, Y.V.V.; Krishnaji, T. Al(OTf)3 as a Highly Efficient Catalyst for the Rapid Acetylation of Alcohols, Phenols and Thiophenols under Solvent-Free Conditions. Tetrahedron Lett. 2007, 48, 3813–3818. [Google Scholar] [CrossRef]
- Das, R.; Chakraborty, D. Silver Triflate Catalyzed Acetylation of Alcohols, Thiols, Phenols, and Amines. Synthesis 2011, 1621–1625. [Google Scholar]
- Rajabi, F. A Heterogeneous Cobalt(II) Salen Complex as an Efficient and Reusable Catalyst for Acetylation of Alcohols and Phenols. Tetrahedron Lett. 2009, 50, 395–397. [Google Scholar] [CrossRef]
- Meshram, G.A.; Patil, V.D. Simple and Efficient Method for Acetylation of Alcohols, Phenols, Amines, and Thiols Using Anhydrous NiCl2 under Solvent-Free Conditions. Synth. Commun. 2009, 39, 4384–4395. [Google Scholar] [CrossRef]
- Mulla, S.A.R.; Inamdar, S.M.; Pathan, M.Y.; Chavan, S.S. Highly Efficient Cobalt (II) Catalyzed O-Acylation of Alcohols and Phenols under Solvent-Free Conditions. Open J. Synth. Theory Appl. 2012, 1, 31–35. [Google Scholar] [CrossRef] [Green Version]
- Phukan, P. Iodine as an extremely powerful catalyst for the acetylation of alcohols under solvent-free conditions. Tetrahedron Lett. 2004, 45, 4785–4787. [Google Scholar] [CrossRef]
- Khan, A.T.; Choudhury, L.H.; Ghosh, S. Acetonyltriphenylphosphonium Bromide (ATPB): A Versatile Reagent for the Acylation of Alcohols, Phenols, Thiols and Amines and for 1,1-Diacylation of Aldehydes under Solvent-Free Conditions. Eur. J. Org. Chem. 2005, 2782–2787. [Google Scholar] [CrossRef]
- Kantam, M.L.; Aziz, K.; Likhar, P.R. Bis(cyclopentadienyl) zirconium dichloride catalyzed acetylation of phenols, alcohols and amines. Catal. Commun. 2006, 7, 484–487. [Google Scholar] [CrossRef]
- Chakraborti, A.K.; Shivani, S. Magnesium Bistrifluoromethanesulfonimide as a New and Efficient Acylation Catalyst. J. Org. Chem. 2006, 71, 5785–5788. [Google Scholar] [CrossRef]
- Kadam, S.T.; Kim, S.S. Phosphomolybdic Acid: Mild and Efficient Catalyst for Acetylation of Alcohols, Phenols, and Amines under Solvent-Free Conditions. Synthesis 2008, 267–268. [Google Scholar] [CrossRef]
- Tale, R.H.; Adude, R.N. A Novel 3-Nitrobenzeneboronic Acid as an Extremely Mild and Environmentally Benign Catalyst for the Acetylation of Alcohols under Solvent-Free Conditions. Tetrahedron Lett. 2006, 47, 7263–7265. [Google Scholar] [CrossRef]
- Sakakura, A.; Kawajiri, K.; Ohkubo, T.; Kosugi, Y.; Ishihara, K. Widely Useful DMAP-Catalyzed Esterification under Auxiliary Base- and Solvent-Free Conditions. J. Am. Chem. Soc. 2007, 129, 14775–14779. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ma, Q.; Liu, Y.; Wang, Q. 4-(N,N-Dimethylamino)pyridine Hydrochloride as a Recyclable Catalyst for Acylation of Inert Alcohols: Substrate Scope and Reaction Mechanism. Org. Lett. 2014, 16, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, A.R.; Karimi, H. Acetylation of alcohols and phenols under solvent-free conditions using copper zirconium phosphate. Chin. J. Catal. 2014, 35, 1982–1989. [Google Scholar] [CrossRef]
- Shirini, F.; Zolfigol, M.A.; Aliakbar, A.-R.; Albadi, J. Efficient Acetylation of Alcohols, Phenols, and Amines Catalyzed by Melamine Trisulfonic Acid (MTSA). Synth. Commun. 2010, 40, 1022–1028. [Google Scholar] [CrossRef]
- Araghi, M.; Mirkhani, V.; Moghadam, M.; Tangestaninejad, S.; Baltork, I.M. New porphyrin-polyoxometalate hybrid materials: Synthesis, characterization and investigation of catalytic activity in acetylation reactions. Dalton Trans. 2012, 41, 11745–11752. [Google Scholar] [CrossRef] [PubMed]
- Mojtahedi, M.M.; Samadian, S. Efficient and Rapid Solvent-Free Acetylation of Alcohols, Phenols, and Thiols Using Catalytic Amounts of Sodium Acetate Trihydrate. J. Chem. 2013. [Google Scholar] [CrossRef]
- Lόpez, I.; Bravo, J.L.; Caraballo, M.; Barneto, J.L.; Silvero, G. Task-Oriented Use of Ionic Liquids: Efficient Acetylation of Alcohols and Phenols. Tetrahedron Lett. 2011, 52, 3339–3341. [Google Scholar] [CrossRef]
- Tamaddon, F.; Amrollahi, M.A.; Sharafat, L. A green protocol for chemoselective O-acylation in the presence of zinc oxide as a heterogeneous, reusable and eco-friendly catalyst. Tetrahedron Lett. 2005, 46, 7841–7844. [Google Scholar] [CrossRef]
- Sarvari, M.H.; Sharghi, H. Zinc Oxide (ZnO) as a New, Highly Efficient, and Reusable Catalyst for Acylation of Alcohols, Phenols and Amines under Solvent Free Conditions. Tetrahedron 2005, 61, 10903–10907. [Google Scholar] [CrossRef]
- Albadi, J.; Alihosseinzadeh, A.; Mardani, M. Efficient approach for the chemoselective acetylation of alcohols catalyzed by a novel metal oxide nanocatalyst CuO-ZnO. Chin. J. Catal. 2015, 36, 308–313. [Google Scholar] [CrossRef]
- Bhosale, M.A.; Ummineni, D.; Sasaki, T.; Hamane, D.N.; Bhanage, B.M. Magnetically separable γ-Fe2O3 nanoparticles: An efficient catalyst for acylation of alcohols, phenols, and amines using sonication energy under solvent free condition. J. Mol. Catal. A: Chem. 2015, 404–405, 8–17. [Google Scholar] [CrossRef]
- Veisi, H.; Taheri, S.; Hemmati, S. Preparation of polydopamine sulfamic acid-functionalized magnetic Fe3O4 nanoparticles with a core/shell nanostructure as heterogeneous and recyclable nanocatalysts for the acetylation of alcohols, phenols, amines and thiols under solvent-free conditions. Green Chem. 2016, 18, 6337–6348. [Google Scholar] [CrossRef]
- Yoon, H.-J.; Lee, S.-M.; Kim, J.-H.; Cho, H.-J.; Choi, J.-W.; Lee, S.-H.; Lee, Y.-S. Polymer-Supported Gadolinium Triflate as a Convenient and Efficient Lewis Acid Catalyst for Acetylation of Alcohols and Phenols. Tetrahedron Lett. 2008, 49, 3165–3171. [Google Scholar] [CrossRef]
- Niknam, K.; Saberi, D. Preparation of sulfuric acid ([3-(3-silicapropyl)sulfanyl]propyl)ester: A new and recyclable catalyst for the formylation and acetylation of alcohols under heterogeneous conditions. Appl. Catal. A Gen. 2009, 366, 220–225. [Google Scholar] [CrossRef]
- Osiglio, L.; Romanelli, G.; Blanco, M. Alcohol acetylation with acetic acid using borated zirconia as catalyst. J. Mol. Catal. A: Chem. 2010, 316, 52–58. [Google Scholar] [CrossRef]
- Farhadi, S.; Panahandehjoo, S. Spinel-Type Zinc Aluminate (ZnAl2O4) Nanoparticles Prepared by the Co-Precipitation Method: A Novel, Green and Recyclable Heterogeneous Catalyst for the Acetylation of Amines, Alcohols and Phenols under Solvent-Free Conditions. Appl. Catal. A Gen. 2010, 382, 293–302. [Google Scholar] [CrossRef]
- Zarei, A.; Hajipour, A.R.; Khazdooz, L. P2O5/Al2O3 as an Efficient Heterogeneous Catalyst for the Acetylation of Alcohols, Phenols, Thiols, and Amines under Solvent-Free Conditions. Synth. Commun. 2011, 41, 1772–1785. [Google Scholar] [CrossRef]
- Khaligh, N.G. Poly(N-vinylimidazole) as an efficient catalyst for acetylation of alcohols, phenols, thiols and amines under solvent-free conditions. RSC Adv. 2013, 3, 99–110. [Google Scholar] [CrossRef]
- Zareyee, D.; Alizadeh, P.; Ghandali, M.S.; Khalilzadeh, M.A. Solvent-free acetylation and tetrahydropyranylation of alcohols catalyzed by recyclable sulfonated ordered nanostructured carbon. Chem. Pap. 2013, 67, 713–721. [Google Scholar] [CrossRef]
- Yu, W.; Zhou, M.; Wang, T.; He, Z.; Shi, B.; Xu, Y.; Huang, K. “Click Chemistry” Mediated Functional Microporous Organic Nanotube Networks for Heterogeneous Catalysis. Org. Lett. 2017, 19, 5776–5779. [Google Scholar] [CrossRef] [PubMed]
- Gade, V.B.; Rathi, A.K.; Bhalekar, S.B.; Tucek, J.; Tomanec, O.; Varma, R.S.; Zboril, R.; Shelke, S.N.; Gawande, M.B. Iron-Oxide-Supported Ultrasmall ZnO Nanoparticles: Applications for Transesterification, Amidation, and O-Acylation Reactions. ACS Sustain. Chem. Eng. 2017, 5, 3314–3320. [Google Scholar] [CrossRef]
- Panahi, F.; Alamdari, R.F.; Dangolani, S.K.; Nezhad, A.K.; Golestanzadeh, M. Graphene Grafted N-Methyl-4-pyridinamine (G-NMPA): An Efficient Heterogeneous Organocatalyst for Acetylation of Alcohols. ChemistrySelect 2017, 2, 474–479. [Google Scholar] [CrossRef]
- Nardi, M.; Luisa Di Gioia, M.; Costanzo, P.; De Nino, A.; Maiuolo, L.; Oliverio, M.; Olivito, F.; Procopio, A. Selective Acetylation of Small Biomolecules and Their Derivatives Catalyzed by Er(OTf)3. Catalysts 2017, 7, 269. [Google Scholar] [CrossRef]
- Nardi, M.; Costanzo, P.; De Nino, A.; Di Gioia, M.L.; Olivito, F.; Sindona, G.; Procopioc, A. Water excellent solvent for the synthesis of bifunctionalized cyclopentenones from furfural. Green Chem. 2017, 19, 5403–5411. [Google Scholar] [CrossRef]
- Di Gioia, M.L.; Costanzo, P.; De Nino, A.; Maiuolo, L.; Nardi, M.; Olivito, F.; Procopio, A. Simple and efficient Fmoc removal in ionic liquid. RSC Adv. 2017, 7, 36482–36491. [Google Scholar] [CrossRef] [Green Version]
- Ranu, B.C.; Dey, S.S.; Hajra, A. Highly efficient acylation of alcohols, amines and thiols under solvent-free and catalyst-free conditions. Green Chem. 2003, 5, 44–46. [Google Scholar] [CrossRef]
- Alleti, R.; Perambuduru, M.; Samantha, S.; Reddy, V.P. Gadolinium triflate: An efficient and convenient catalyst for acetylation of alcohols and amines. J. Mol. Catal. A: Chem. 2005, 226, 57–59. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, Y.; Fan, R.; Wu, J. Metal- and solvent-free conditions for the acylation reaction catalyzed by carbon tetrabromide (CBr4). Green Chem. 2007, 9, 1022–1025. [Google Scholar] [CrossRef]
- Chakraborti, A.K.; Gulhane, R. Indium(III) chloride as a new, highly efficient, and versatile catalyst for acylation of phenols, thiols, alcohols, and amines. Tetrahedron Lett. 2003, 44, 6749–6753. [Google Scholar] [CrossRef]
- Singh, S.J.; Kale, S.R.; Gawande, M.B.; Velhinho, A.; Jayaram, R.V. A synthesis of copper based metal-organic framework for O-acetylation of alcohols. Catal. Commun. 2014, 44, 24–28. [Google Scholar] [CrossRef]
- Heravi, M.M.; Behbahani, F.K.; Bamoharram, F.F. H14[NaP5W30O110]: A heteropoly acid catalyzed acetylation of alcohols and phenols in acetic anhydride. J. Mol. Catal. A Chem. 2006, 253, 16–19. [Google Scholar] [CrossRef]
- Nakae, Y.; Kusaki, I.; Sato, T. Lithium Perchlorate Catalyzed Acetylation of Alcohols under Mild Reaction Conditions. Synlett 2001, 1584–1586. [Google Scholar] [CrossRef]



| Entry | Substrate | Product | Time (h) | Conv. b (%) | Sel. b (%) | Isolated Yield (%) |
|---|---|---|---|---|---|---|
| 1 |  |  | 24 | 63 c | 100 | 60 |
| 7 | 100 | 100 | 98 | |||
| 7 | 88 d | 100 | 82 | |||
| 2 |  |  | 8 | >99 | 100 | 96 |
| 3 |  |  | 8 | >99 | 100 | 96 |
| 4 |  |  | 8 | >99 | 100 | 96 |
| 5 |  |  | 12 | >99 | 100 | 95 |
| 16 | >99 f | 100 | 96 | |||
| 6 |  |  | 7 | 100 | 100 | 97 |
| 7 |  |  | 7 | 100 | 100 | 98 |
| 8 |  |  | 8 | >99 | 100 | 96 |
| 9 |  |  | 20 | >99 e | 100 | 95 |
| 10 |  |  | 20 | >98 e | 100 | 95 |
| 11 |  |  | 12 | >99 e | 100 | 96 |
| 12 |  |  | 20 | >99 | 100 | 95 |
| 13 |  |  | 20 | >99 | 100 | 97 |
| 14 |  |  | 20 | 99 | 100 | 96 |
| 24 | 97f | 100 | 95 | |||
| 15 |  |  | 20 | >98 | 100 | 95 |
| 16 |  |  | 20 | >99 e | 100 | 98 |
| 17 |  |  | 0.5 | 100 | 100 | 97 |
| 18 |  |  | 0.5 | 100 | 100 | 98 |
| 19 |  |  | 0.5 | 100 | 100 | 98 |
| 0.5 | 100 f | 100 | 98 | |||
| 20 |  |  | 0.5 | 100 | 100 | 97 |
| 21 |  |  | 0.5 | 100 | 100 | 96 |
| 22 |  |  | 4 | 100 | 98 | 94 |
| 15 | 99 e,f | 98 | 96 | |||
| 23 |  |  | 6 | 100 | 97 | 95 |
| 24 |  |  | 20 | 86 e | 97 | 80 |
| Entry | Substrate (mmol) | (CH3CO)2O (mmol) | Catalyst | Solvent | T (°C) | Time (h) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 2 | Cu(OTf)2 | DCM | RT | 2 | [11] |
| 2 | 1 | 1.2 | RuCl3 | CH3CN | RT | 10 min–72 h | [18] |
| 3 | 1 | 1.5–2 | Ph3P+CH2COMeBr− | - | RT | 0.5–3.5 | [28] |
| 4 | 55.5 | 83 | Gd(OTf)3 | CH3CN | 25 | 5 min–14 h | [59] |
| 5 | 1 | 5 | Co(II)salen-complex | - | 50 | 0.5–2 | [24] |
| 6 | 5 | 6 | CoCl2 | - | RT | 10–50 min | [26] |
| 7 | 1 | 1.1 | NaOAc⋅3H2O | - | RT | 10 min | [38] |
| 8 | 10 | 11 | DMAP·HCl | Toluene | RT-110 | 4–28 | [34] |
| 9 | 0.1 | 0.15 | DMAP-MONN a | CH2Cl2 | RT | 0.5–5 | [52] |
| 10 | 6.9 | 7.6 | Maghemite-ZnO | - | RT | 3 | [53] |
| 11 | 1 | 1.5 | G-NMPA a | - | 35 | 2–10 | [54] |
| 12 | 0.5 | 0.55 | CBr4 | - | 60 | 3–6 | [60] |
| 13 | 2.5 | 2.5 | InCl3 | - | RT | 30 min | [61] |
| 14 | 2 | 4 | Cu(BDC) | - | RT | 24 | [62] |
| 15 | 1 | 1.5 | H14[NaP5W30O110] | - | RT | 0.5–3 | [63] |
| 16 | 2 | 4–20 | LiClO4 | - | 25–40 | 4–48 | [64] |
| 17 | 1 | 1.5 | - | - | 60–70 | 7–20 | Present work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anbu, N.; Nagarjun, N.; Jacob, M.; Kalaiarasi, J.M.V.K.; Dhakshinamoorthy, A. Acetylation of Alcohols, Amines, Phenols, Thiols under Catalyst and Solvent-Free Conditions. Chemistry 2019, 1, 69-79. https://doi.org/10.3390/chemistry1010006
Anbu N, Nagarjun N, Jacob M, Kalaiarasi JMVK, Dhakshinamoorthy A. Acetylation of Alcohols, Amines, Phenols, Thiols under Catalyst and Solvent-Free Conditions. Chemistry. 2019; 1(1):69-79. https://doi.org/10.3390/chemistry1010006
Chicago/Turabian StyleAnbu, Nagaraj, Nagarathinam Nagarjun, Manju Jacob, J. Mary Vimala Kumari Kalaiarasi, and Amarajothi Dhakshinamoorthy. 2019. "Acetylation of Alcohols, Amines, Phenols, Thiols under Catalyst and Solvent-Free Conditions" Chemistry 1, no. 1: 69-79. https://doi.org/10.3390/chemistry1010006