Electrochemical Behavior of Pt–Ru Catalysts Supported on Graphitized Ordered Mesoporous Carbons toward CO and Methanol Oxidation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Graphitized Ordered Mesoporous Carbon (gCMK)
2.2. Synthesis of gCMK-3-Supported Pt and Pt–Ru Catalysts
2.3. Physicochemical Characterization of the Catalysts
2.4. Electrochemical Characterization
3. Results
3.1. Physical Characterization
3.2. CO Stripping
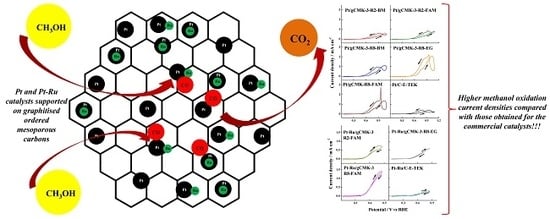
3.3. Methanol Oxidation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lu, A.; Schüth, F. Nanocasting: A Versatile strategy for creating nanostructured porous materials. Adv. Mater. 2006, 18, 1793–1805. [Google Scholar] [CrossRef]
- Joo, S.H.; Choi, S.J.; Oh, I.; Kwak, J.; Liu, Z.; Terasaki, O.; Ryoo, R. Ordered nanoporous arrays of carbon supporting high dispersions of platinum nanoparticles. Nature 2001, 412, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Calvillo, L.; Lázaro, M.J.; García-Bordejé, E.; Moliner, R.; Cabot, P.L.; Esparbé, I.; Pastor, E.; Quintana, J.J. Platinum supported on functionalized ordered mesoporous carbon as electrocatalyst for direct methanol fuel cells. J. Power Sources 2007, 169, 59–64. [Google Scholar] [CrossRef]
- Salgado, J.R.C.; Quintana, J.J.; Calvillo, L.; Lázaro, M.J.; Cabot, P.L.; Esparbé, I.; Pastor, E. Carbon monoxide and methanol oxidation at platinum catalysts supported on ordered mesoporous carbon: The influence of functionalization of the support. Phys. Chem. Chem. Phys. 2008, 10, 6796–6806. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; An, L.; Dang, L.; Zhao, M.; Shi, J.; Bai, S.; Cao, Y. Highly dispersed platinum supported on nitrogen-containing ordered mesoporous carbon for methanol electrochemical oxidation. Microporous Mesoporous Mater. 2009, 119, 30–38. [Google Scholar] [CrossRef]
- Calvillo, L.; Celorrio, V.; Moliner, R.; García, A.B.; Camean, I.; Lázaro, M.J. Comparative study of Pt catalysts supported on different high conductive carbon materials for methanol and ethanol oxidation. Electrochim. Acta 2013, 102, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Eftekhari, A.; Fan, Z. Ordered mesoporous carbon and its applications for electrochemical energy storage and conversion. Mater. Chem. Front. 2017, 1, 1001–1027. [Google Scholar] [CrossRef]
- Fulvio, P.F.; Mayes, R.T.; Wang, X.; Mahurin, S.M.; Bauer, J.C.; Presser, V.; McDonough, J.; Gogotsi, Y.; Dai, S. “Brick-and-mortar” self-assembly approach to graphitic mesoporous carbon nanocomposites. Adv. Funct. Mater. 2011, 21, 2208–2215. [Google Scholar] [CrossRef]
- Kim, C.H.; Lee, D.K.; Pinnavaia, T.J. Graphitic mesostructured carbon prepared from aromatic precursors. Langmuir 2004, 20, 5157–5159. [Google Scholar] [CrossRef]
- Lee, K.; Ji, X.; Rault, M.; Nazar, L. Simple synthesis of graphitic ordered mesoporous carbon materials by a solid-state method using metal phthalocyanines. Angew. Chem. 2009, 48, 5661–5665. [Google Scholar] [CrossRef]
- Fuertes, A.B.; Alvarez, S. Graphitic mesoporous carbons synthesised through mesostructured silica templates. Carbon 2004, 42, 3049–3055. [Google Scholar] [CrossRef]
- Tang, J.; Wang, T.; Sun, X.; Guo, Y.; Xue, H.; Guo, H.; Liu, M.; Zhang, X.; He, J. Effect of transition metal on catalytic graphitization of ordered mesoporous carbon and Pt/metal oxide synergistic electrocatalytic performance. Microporous Mesoporous Mater. 2013, 177, 105–112. [Google Scholar] [CrossRef]
- Nettelroth, D.; Schwarz, H.C.; Burblies, N.; Guschanski, N.; Behrens, P. Catalytic graphitization of ordered mesoporous carbon CMK-3 with iron oxide catalysts: Evaluation of different synthesis pathways. Phys. Status Solidi A 2016, 213, 1395–1402. [Google Scholar] [CrossRef]
- Sultana, K.N.; Fadhel, A.L.; Deshmane, V.G.; Ilias, S. Novel method for synthesis of electrocatalyst via catalytic graphitization of ordered mesoporous carbon for PEMFC application. Sep. Sci. Technol. 2018, 53, 1948–1956. [Google Scholar] [CrossRef]
- Celorrio, V.; Sebastián, D.; Calvillo, L.; García, A.B.; Fermín, D.J.; Lázaro, M.J. Influence of thermal treatments on the stability of Pd nanoparticles supported on graphitised ordered mesoporous carbons. Int. J. Hydrog. Energy 2016, 41, 19570–19578. [Google Scholar] [CrossRef] [Green Version]
- Rivera Gavidia, L.M.; García, G.; Celorrio, V.; Lázaro, M.J.; Pastor, E. Methanol tolerant Pt2CrCo catalysts supported on ordered mesoporous carbon for the cathode of DMFC. Int. J. Hydrog. Energy 2016, 41, 19645–19655. [Google Scholar] [CrossRef]
- Calvillo, L.; Celorrio, V.; Moliner, R.; Cabot, P.L.; Esparbé, I.; Lázaro, M.J. Control of textural properties of ordered mesoporous materials. Microporous Mesoporous Mater. 2008, 116, 292–298. [Google Scholar] [CrossRef]
- Lázaro, M.J.; Calvillo, L.; Bordejé, E.G.; Moliner, R.; Juan, R.; Ruiz, C.R. Functionalization of ordered mesoporous carbons synthesized with SBA-15 silica as template. Microporous Mesoporous Mater. 2007, 103, 158–165. [Google Scholar] [CrossRef]
- Wang, X.; Hsing, I.M. Surfactant stabilized Pt and Pt alloy electrocatalyst for polymer electrolyte fuel cells. Electrochim. Acta 2002, 47, 2981–2987. [Google Scholar] [CrossRef]
- Warren, B.E. X-ray Diffraction, 1st ed.; Addison-Wesley: Reading, UK, 1969; pp. 27–40. ISBN 0486663175. [Google Scholar]
- Calderón, J.C.; García, G.; Calvillo, L.; Rodríguez, J.L.; Lázaro, M.J.; Pastor, E. Electrochemical oxidation of CO and methanol on Pt–Ru catalysts supported on carbon nanofibers: The influence of synthesis method. Appl. Catal. B-Environ. 2015, 165, 676–686. [Google Scholar] [CrossRef]
- Calderón, J.C.; Mahata, N.; Pereira, M.F.R.; Figueiredo, J.L.; Fernandes, V.R.; Rangel, C.M.; Calvillo, L.; Lázaro, M.J.; Pastor, E. Pt-Ru catalysts supported on carbon xerogels for PEM fuel cells. Int. J. Hydrog. Energy 2012, 37, 7200–7211. [Google Scholar] [CrossRef]
- Sebastián, D.; Calderón, J.C.; González-Expósito, J.A.; Pastor, E.; Martínez-Huerta, M.V.; Suelves, I.; Moliner, R.; Lázaro, M.J. Influence of carbon nanofiber properties as electrocatalyst support on the electrochemical performance for PEM fuel cells. Int. J. Hydrog. Energy 2010, 35, 9934–9942. [Google Scholar] [CrossRef]
- Calderón, J.C.; Ndzuzo, L.; Bladergroen, B.J.; Pasupathi, S. Oxygen reduction reaction on Pt-Pd catalysts supported on carbon xerogels: Effect of the synthesis method. Int. J. Hydrog. Energy 2018, 43, 16881–16896. [Google Scholar] [CrossRef]
- Yang, G.; Sun, Y.; Lv, P.; Zhen, F.; Cao, X.; Chen, X.; Wang, Z.; Yuan, Z.; Kong, X. Preparation of Pt–Ru/C as an oxygen-reduction electrocatalyst in microbial fuel cells for wastewater treatment. Catalysts 2016, 6, 150. [Google Scholar] [CrossRef]
- Ye, F.; Liu, H.; Hu, W.; Zhong, J.; Chen, Y.; Cao, H.; Yang, J. Heterogeneous Au-Pt nanostructures with enhanced catalytic activity toward oxygen reduction. Dalton Trans. 2012, 41, 2898–2903. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Liu, H.; Ye, F.; Hu, W.; Yang, J. Cage-bell structured Au-Pt nanomaterials with enhanced electrocatalytic activity toward oxygen reduction. Int. J. Hydrog. Energy 2012, 37, 13191–13199. [Google Scholar] [CrossRef]
- Kim, G.J.; Kwon, D.W.; Hong, S.C. Effect of Pt particle size and valence state on the performance of Pt/TiO2 catalysts for CO oxidation at room temperature. J. Phys. Chem. C 2016, 120, 17996–18004. [Google Scholar] [CrossRef]
- Jeon, T.Y.; Lee, K.S.; Yoo, S.J.; Cho, Y.H.; Kang, S.H.; Sung, Y.E. Effect of surface segregation on the methanol oxidation reaction in carbon-supported Pt−Ru alloy nanoparticles. Langmuir 2010, 26, 9123–9129. [Google Scholar] [CrossRef]
- Salgado, J.R.C.; Alcaide, F.; Álvarez, G.; Calvillo, L.; Lázaro, M.J.; Pastor, E. Pt–Ru electrocatalysts supported on ordered mesoporous carbon for direct methanol fuel cell. J. Power Sources 2010, 195, 4022–4029. [Google Scholar] [CrossRef]
- Chung, D.Y.; Lee, K.J.; Sung, Y.E. Methanol electro-oxidation on the Pt surface: Revisiting the cyclic voltammetry interpretation. J. Phys. Chem. C 2016, 120, 9028–9035. [Google Scholar] [CrossRef]
- Fu, X.; Zhao, Z.; Wan, C.; Wang, Y.; Fan, Z.; Song, F.; Cao, B.; Li, M.; Xue, W.; Huang, Y.; et al. Ultrathin wavy Rh nanowires as highly effective electrocatalysts for methanol oxidation reaction with ultrahigh ECSA. Nano Res. 2019, 12, 211–215. [Google Scholar] [CrossRef]
- Calderón, J.C.; García, G.; Querejeta, A.; Alcaide, F.; Calvillo, L.; Lázaro, M.J.; Rodríguez, J.L.; Pastor, E. Carbon monoxide and methanol oxidations on carbon nanofibers supported Pt–Ru electrodes at different temperatures. Electrochim. Acta 2015, 186, 359–368. [Google Scholar] [CrossRef]
- Su, F.; Zeng, J.; Bao, X.; Yu, Y.; Lee, J.Y.; Zhao, X.S. Preparation and characterization of highly ordered graphitic mesoporous carbon as a Pt catalyst support for direct methanol fuel cells. Chem. Mater. 2005, 17, 3960–3967. [Google Scholar] [CrossRef]
- Maiyalagan, T.; Alaje, T.O.; Scott, K. Highly stable Pt-Ru nanoparticles supported on three-dimensional cubic ordered mesoporous carbon (Pt-Ru/CMK-8) as promising electrocatalysts for methanol oxidation. J. Phys. Chem. C 2012, 116, 2630–2638. [Google Scholar] [CrossRef]
- Sebastián, D.; Nieto-Monge, M.J.; Pérez-Rodríguez, S.; Pastor, E.; Lázaro, M.J. Nitrogen doped ordered mesoporous carbon as support of PtRu nanoparticles for methanol electro-oxidation. Energies 2018, 11, 831. [Google Scholar] [CrossRef]









| Catalyst | Metal Content, wt % | Pt:Ru Atomic Ratio | Crystallite and Particle Size, nm | Lattice Parameter, Å | Metal Surface Area, m2 g−1 | |
|---|---|---|---|---|---|---|
| XRD | TEM | |||||
| Pt/gCMK-3-R2-BM | 20 | ----- | 5.9 | 3.9 ± 1.3 | 3.923 | 47 |
| Pt/gCMK-3-R2-FAM | 22 | ----- | 3.7 | 3.2 ± 1.0 | 3.926 | 76 |
| Pt/gCMK-3-R8-BM | 16 | ----- | 5.6 | 4.1 ± 1.4 | 3.920 | 50 |
| Pt/gCMK-3-R8-EG | 24 | ----- | 6.2 | 5.9 ± 1.4 | 3.919 | 45 |
| Pt/gCMK-3-R8-FAM | 23 | ----- | 4.1 | 4.9 ± 1.6 | 3.920 | 68 |
| Pt/C E-TEK | 20 | ----- | 3.0 | ----- | 3.921 | 93 |
| Pt–Ru/gCMK-3-R2-FAM | 26 | 53:47 | 6.6 | 5.2 ± 2.3 | 3.896 | 49 |
| Pt–Ru/gCMK-3-R8-EG | 22 | 67:33 | 3.6 | 6.7 ± 2.8 | 3.921 | 85 |
| Pt–Ru/gCMK-3-R8-FAM | 24 | 55:45 | 5.6 | 3.9 ± 1.3 | 3.898 | 57 |
| Pt–Ru/C E-TEK | 20 | 45:55 | 4.4 | ----- | 3.866 | 76 |
| Catalyst | Chemical State | Relative Area, % | Binding Energy, eV |
|---|---|---|---|
| Pt/gCMK-3-R2-BM | Pt 4f 7/2 | ||
| Metallic Pt | 59.4 | 71.4 | |
| Pt2+ | 21.7 | 72.6 | |
| Pt4+ | 18.9 | 75.3 | |
| Pt/gCMK-3-R2-FAM | Pt 4f 7/2 | ||
| Metallic Pt | 60.9 | 71.4 | |
| Pt2+ | 20.6 | 72.6 | |
| Pt4+ | 18.5 | 75.3 | |
| Pt/gCMK-3-R8-BM | Pt 4f 7/2 | ||
| Metallic Pt | 61.1 | 71.4 | |
| Pt2+ | 19.5 | 72.6 | |
| Pt4+ | 19.4 | 75.3 | |
| Pt/gCMK-3-R8-EG | Pt 4f 7/2 | ||
| Metallic Pt | 61.7 | 71.4 | |
| Pt2+ | 20.1 | 72.6 | |
| Pt4+ | 18.2 | 75.4 | |
| Pt/gCMK-3-R8-FAM | Pt 4f 7/2 | ||
| Metallic Pt | 62.1 | 71.5 | |
| Pt2+ | 18.8 | 72.7 | |
| Pt4+ | 19.1 | 75.5 | |
| Pt–Ru/gCMK-3-R2-FAM | Pt 4f 7/2 | ||
| Metallic Pt | 28.7 | 71.4 | |
| Pt2+ | 17.4 | 72.6 | |
| Pt4+ | 53.9 | 74.2 | |
| Ru 3p 3/2 | |||
| Metallic Ru | 14.9 | 461.0 | |
| Ru4+ | 27.9 | 463.2 | |
| Ru4+ hydrate | 57.2 | 465.4 | |
| Pt–Ru/gCMK-3-R8-EG | Pt 4f 7/2 | ||
| Metallic Pt | 59.1 | 71.4 | |
| Pt2+ | 22.2 | 72.7 | |
| Pt4+ | 18.7 | 75.7 | |
| Ru 3p 3/2 | |||
| Metallic Ru | 43.6 | 461.6 | |
| Ru4+ | 18.6 | 463.2 | |
| Ru4+ hydrate | 37.8 | 465.3 | |
| Pt–Ru/gCMK-3-R8-FAM | Pt 4f 7/2 | ||
| Metallic Pt | 63.5 | 71.6 | |
| Pt2+ | 19.2 | 72.9 | |
| Pt4+ | 17.28 | 75.4 | |
| Ru 3p 3/2 | |||
| Metallic Ru | 18.9 | 461.1 | |
| Ru4+ | 40.7 | 463.3 | |
| Ru4+ hydrate | 40.4 | 465.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderón Gómez, J.C.; Celorrio, V.; Calvillo, L.; Sebastián, D.; Moliner, R.; Lázaro Elorri, M.J. Electrochemical Behavior of Pt–Ru Catalysts Supported on Graphitized Ordered Mesoporous Carbons toward CO and Methanol Oxidation. Surfaces 2019, 2, 1-15. https://doi.org/10.3390/surfaces2010001
Calderón Gómez JC, Celorrio V, Calvillo L, Sebastián D, Moliner R, Lázaro Elorri MJ. Electrochemical Behavior of Pt–Ru Catalysts Supported on Graphitized Ordered Mesoporous Carbons toward CO and Methanol Oxidation. Surfaces. 2019; 2(1):1-15. https://doi.org/10.3390/surfaces2010001
Chicago/Turabian StyleCalderón Gómez, Juan Carlos, Verónica Celorrio, Laura Calvillo, David Sebastián, Rafael Moliner, and María Jesús Lázaro Elorri. 2019. "Electrochemical Behavior of Pt–Ru Catalysts Supported on Graphitized Ordered Mesoporous Carbons toward CO and Methanol Oxidation" Surfaces 2, no. 1: 1-15. https://doi.org/10.3390/surfaces2010001