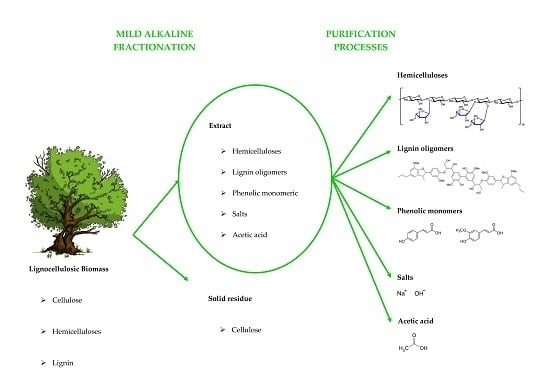
Lignocellulosic Biomass Mild Alkaline Fractionation and Resulting Extract Purification Processes: Conditions, Yields, and Purities
Abstract
:1. Introduction
2. Mild Alkaline Fractionation
2.1. Effect and Mechanism
2.2. Nature of the Base
2.3. Conditions and Yields
2.4. Industrial Applications
3. Purification Routes Applied to Alkaline Hydrolysates
3.1. Flocculation
3.2. Precipitation
3.2.1. Acidification
3.2.2. Ethanol Addition
3.3. Adsorption
3.3.1. Activated Charcoal
3.3.2. Resin
3.4. Low-Pressure Chromatography
3.5. Cross-Flow Membrane Filtration
3.6. Electrodialysis
3.7. Combination of Different Purification Techniques
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Balan, V. Current challenges in commercially producing biofuels from lignocellulosic biomass. ISRN Biotechnol. 2014, 2014, 463074. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef] [PubMed]
- Cardona, C.A.; Quintero, J.A.; Paz, I.C. Production of bioethanol from sugarcane bagasse: Status and perspectives. Bioresour. Technol. 2010, 101, 4754–4766. [Google Scholar] [CrossRef] [PubMed]
- Oriez, V.; Peydecastaing, J.; Pontalier, P.-Y. Lignocellulosic biomass fractionation by mineral acids and resulting extract purification processes: Conditions, yields, and purities. Molecules 2019, 24, 4273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, J.W. Improved Process for Making Paper-Pulp [Internet]. U.S. Patent 51572A, 28 September 1948. Available online: https://patents.google.com/patent/US51572A/en (accessed on 18 September 2018).
- Dahl, G.F. Process of Manufacturing Cellulose from Wood [Internet]. U.S. Patent 296935A, 15 April 1884. Available online: https://patents.google.com/patent/US296935A/en (accessed on 18 September 2018).
- Eckert, R.C.; Abdullah, Z. Carbon Fibers from Kraft Softwood Lignin [Internet]. U.S. Patent 20080318043 A1, 25 December 2008. Available online: http://www.google.fr/patents/US20080318043 (accessed on 4 September 2015).
- Cardoso, M.; de Oliveira, É.D.; Passos, M.L. Chemical composition and physical properties of black liquors and their effects on liquor recovery operation in Brazilian pulp mills. Fuel 2009, 88, 756–763. [Google Scholar] [CrossRef]
- Christensen, T. A Mathematical Model of the Kraft Pulping Process. Bachelor’s Thesis, The Purdue University, West Lafayette, IN, USA, 1982; pp. 1–535. [Google Scholar]
- Phillips, M. Benjamin Chew Tilghman, and the origin of the sulfite process for delignification of wood. J. Chem. Educ. 1943, 20, 444. [Google Scholar] [CrossRef]
- Pokhrel, D.; Viraraghavan, T. Treatment of pulp and paper mill wastewater—A review. Sci. Total Environ. 2004, 333, 37–58. [Google Scholar] [CrossRef]
- Biermann, C.J. Essentials of Pulping and Papermaking; Academic Press: San Diego, CA, USA, 1993; p. 472. [Google Scholar]
- Niemelä, K.; Sjöström, E. Simultaneous identification of aromatic and aliphatic low molecular weight compounds from alkaline pulping liquor by capillary gas-liquid chromatography—Mass spectrometry. Holzforsch. Int. J. Biol. Chem. Phys. Technol. Wood 1986, 40, 361–368. [Google Scholar] [CrossRef]
- Uloth, V.C.; Wearing, J.T. Kraft lignin recovery: Acid precipitation versus ultrafiltration. I. Laboratory Test Results. Pulp Pap Can [Internet]. 1989. Available online: http://agris.fao.org/agris-search/search.do?recordID=US201302658976 (accessed on 23 February 2016).
- Hayes, D.J. An examination of biorefining processes, catalysts and challenges. Catal. Today 2009, 145, 138–151. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.C.; Cotta, M.A. Enzymatic saccharification and fermentation of alkaline peroxide pretreated rice hulls to ethanol. Enzyme Microb. Technol. 2007, 41, 528–532. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G.; Aden, A.; Bozell, J.; Holladay, J.; White, J.; Elliot, D.; Lasure, L.; Jones, S.; Gerber, M.; et al. Top Value Added Chemicals From Biomass: Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; U.S. Department of Energy: Oak Ridge, TN, USA, 2004.
- Ruiz, H.A.; Cerqueira, M.A.; Silva, H.D.; Rodríguez-Jasso, R.M.; Vicente, A.A.; Teixeira, J.A. Biorefinery valorization of autohydrolysis wheat straw hemicellulose to be applied in a polymer-blend film. Carbohydr. Polym. 2013, 92, 2154–2162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, L.; Du, M.; Zhang, J. Hemicellulose-based hydrogels present status and application prospects: A brief review. Open J. For. 2017, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Holladay, J.; Bozell, J.; White, J.; Johnson, D. Top Value Added Chemicals from Biomass: Volume II—Results of Screening for Potential Candidates from Biorefinery Lignin; U.S. Department of Energy: Oak Ridge, TN, USA, 2007.
- Norgren, M.; Edlund, H. Lignin: Recent advances and emerging applications. Curr. Opin. Colloid Interface Sci. 2014, 19, 409–416. [Google Scholar] [CrossRef]
- El Mansouri, N.-E.E.; Salvadó, J. Structural characterization of technical lignins for the production of adhesives: Application to lignosulfonate, kraft, soda-anthraquinone, organosolv and ethanol process lignins. Ind. Crops Prod. 2006, 24, 8–16. [Google Scholar] [CrossRef]
- Ou, S.; Luo, Y.; Xue, F.; Huang, C.; Zhang, N.; Liu, Z. Seperation and purification of ferulic acid in alkaline-hydrolysate from sugarcane bagasse by activated charcoal adsorption/anion macroporous resin exchange chromatography. J. Food Eng. 2007, 78, 1298–1304. [Google Scholar] [CrossRef]
- Ou, S.Y.; Luo, Y.L.; Huang, C.H.; Jackson, M. Production of coumaric acid from sugarcane bagasse. Innov. Food Sci. Emerg. Technol. 2009, 10, 253–259. [Google Scholar] [CrossRef]
- Tilay, A.; Bule, M.; Kishenkumar, J.; Annapure, U. Preparation of ferulic acid from agricultural wastes: Its improved extraction and purification. J. Agric. Food Chem. 2008, 56, 7644–7648. [Google Scholar] [CrossRef]
- Torre, P.; Aliakbarian, B.; Rivas, B.; Domínguez, J.M.; Converti, A. Release of ferulic acid from corn cobs by alkaline hydrolysis. Biochem. Eng. J. 2008, 40, 500–506. [Google Scholar] [CrossRef]
- Chang, M.; Li, D.; Wang, W.; Chen, D.; Zhang, Y.; Hu, H.; Ye, X. Comparison of sodium hydroxide and calcium hydroxide pretreatments on the enzymatic hydrolysis and lignin recovery of sugarcane bagasse. Bioresour. Technol. 2017, 244, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- Oriez, V.; Beyerle, M.; Pontalier, P.-Y.; Peydecastaing, J. Sugarcane bagasse mild alkaline fractionation and production of purified fractions by pulse chromatography with water. Ind. Crops Prod. 2018, 125, 370–378. [Google Scholar] [CrossRef] [Green Version]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Arakane, M.; Ike, M.; Wada, M.; Takai, T.; Gau, M.; Tokuyasu, K. Low temperature alkali pretreatment for improving enzymatic digestibility of sweet sorghum bagasse for ethanol production. Bioresour. Technol. 2011, 102, 4793–4799. [Google Scholar] [CrossRef]
- Kim, S.; Holtzapple, M.T. Delignification kinetics of corn stover in lime pretreatment. Bioresour. Technol. 2006, 97, 778–785. [Google Scholar] [CrossRef]
- Sun, R.; Lawther, J.M.; Banks, W.B. Influence of alkaline pre-treatments on the cell wall components of wheat straw. Ind. Crops Prod. 1995, 4, 127–145. [Google Scholar] [CrossRef]
- Bunzel, M.; Ralph, J.; Kim, H.; Lu, F.; Ralph, S.A.; Marita, J.M.; Hatfield, R.D.; Steinhart, H. Sinapate dehydrodimers and sinapate−ferulate heterodimers in cereal dietary fiber. J. Agric. Food Chem. 2003, 51, 1427–1434. [Google Scholar] [CrossRef]
- Harmsen, P.F.H.; Huijen, W.J.J.; Bermúdez López, L.M.; Bakker, R.R.C. Literature Review of Physical and Chemical Pretreatment Processes for Lignocellulosic Biomass; Report No.: ECN-E-10-013; Wageningen University, Energy Research Centre of The Netherlands: Petten, The Netherlands, 2010. [Google Scholar]
- Sun, R.; Fang, J.; Rowlands, P. Physico-chemical and thermal characterization of alkali-soluble lignins from wheat straw. Polym. J. 1998, 30, 289–294. [Google Scholar] [CrossRef] [Green Version]
- Sun, R.C.; Tomkinson, J. Essential Guides for Isolation/Purification of Polysaccharides. Encycl. Sep. Sci. 2000, 6, 4568–4574. [Google Scholar]
- Sun, J.X.; Sun, X.F.; Sun, R.C.; Su, Y.Q. Fractional extraction and structural characterization of sugarcane bagasse hemicelluloses. Carbohydr. Polym. 2004, 56, 195–204. [Google Scholar] [CrossRef]
- Lora, J.H.; Glasser, W.G. Recent industrial applications of lignin: A sustainable alternative to nonrenewable materials. J. Polym. Environ. 2002, 10, 39–48. [Google Scholar] [CrossRef]
- Sun, R.; Tomkinson, J.; Bolton, J. Effects of precipitation pH on the physico-chemical properties of the lignins isolated from the black liquor of oil palm empty fruit bunch fibre pulping. Polym. Degrad. Stab. 1999, 63, 195–200. [Google Scholar] [CrossRef]
- Sun, R.; Sun, X.F.; Wang, S.Q.; Zhu, W.; Wang, X.Y. Ester and ether linkages between hydroxycinnamic acids and lignins from wheat, rice, rye, and barley straws, maize stems, and fast-growing poplar wood. Ind. Crops Prod. 2002, 15, 179–188. [Google Scholar] [CrossRef]
- Xu, F.; Sun, R.-C.; Sun, J.-X.; Liu, C.-F.; He, B.-H.; Fan, J.-S. Determination of cell wall ferulic and p-coumaric acids in sugarcane bagasse. Anal. Chim. Acta 2005, 552, 207–217. [Google Scholar] [CrossRef]
- Hu, L.; Pan, H.; Zhou, Y.; Zhang, M. Methods to improve lignin’s reactivity as a phenol subsitute and as replacement for other phenolic compounds: A brief review. BioResources 2011, 6, 3515–3525. [Google Scholar]
- Chaturvedi, V.; Verma, P. An overview of key pretreatment processes employed for bioconversion of lignocellulosic biomass into biofuels and value added products. 3 Biotech 2013, 3, 415–431. [Google Scholar] [CrossRef] [Green Version]
- Peng, F.; Peng, P.; Xu, F.; Sun, R.-C. Fractional purification and bioconversion of hemicelluloses. Biotechnol. Adv. 2012, 30, 879–903. [Google Scholar] [CrossRef]
- Chang, V.S.; Nagwani, M.; Holtzapple, M.T. Lime pretreatment of crop residues bagasse and wheat straw. Appl. Biochem. Biotechnol. 1998, 74, 135–159. [Google Scholar] [CrossRef]
- Grimaldi, M.P.; Marques, M.P.; Laluce, C.; Cilli, E.M.; Sponchiado, S.R.P. Evaluation of Lime and Hydrothermal Pretreatments for Efficient Enzymatic hydrolysis of Raw Sugarcane Bagasse. Biotechnol Biofuels [Internet]. 2015. Available online: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4667421/ (accessed on 18 May 2016).
- Kim, T.H.; Taylor, F.; Hicks, K.B. Bioethanol production from barley hull using SAA (soaking in aqueous ammonia) pretreatment. Bioresour. Technol. 2008, 99, 5694–5702. [Google Scholar] [CrossRef]
- Da Silva, C.G.; Grelier, S.; Pichavant, F.; Frollini, E.; Castellan, A. Adding value to lignins isolated from sugarcane bagasse and Miscanthus. Ind. Crops Prod. 2013, 42, 87–95. [Google Scholar] [CrossRef]
- Saha, B.C.; Cotta, M.A. Lime pretreatment, enzymatic saccharification and fermentation of rice hulls to ethanol. Biomass Bioenergy 2008, 32, 971–977. [Google Scholar] [CrossRef]
- Vancov, T.; McIntosh, S. Alkali pretreatment of cereal crop residues for second-generation biofuels. Energy Fuels 2011, 25, 2754–2763. [Google Scholar] [CrossRef]
- Peng, F.; Ren, J.-L.; Xu, F.; Bian, J.; Peng, P.; Sun, R.-C. Comparative study of hemicelluloses obtained by graded ethanol precipitation from sugarcane bagasse. J. Agric. Food Chem. 2009, 57, 6305–6317. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-X.; Sun, X.-F.; Sun, R.-C.; Fowler, P.; Baird, M.S. Inhomogeneities in the chemical structure of sugarcane bagasse lignin. J. Agric. Food Chem. 2003, 51, 6719–6725. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Nie, S.; Yuan, Y.; Wang, S.; Qin, C. Efficient extraction of bagasse hemicelluloses and characterization of solid remainder. Bioresour. Technol. 2015, 185, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Aita, G.; Day, D.F. Compositional changes in sugarcane bagasse on low temperature, long-term diluted ammonia treatment. Appl. Biochem. Biotechnol. 2010, 161, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.K.; Antunes, F.A.; Silva, M.B.; da Silva, S.S. Unraveling the structure of sugarcane bagasse after soaking in concentrated aqueous ammonia (SCAA) and ethanol production by Scheffersomyces (Pichia) stipitis. Biotechnol. Biofuels 2013, 6, 102. [Google Scholar] [CrossRef] [Green Version]
- Xiao, B.; Sun, X.F.; Sun, R. Chemical, structural, and thermal characterizations of alkali-soluble lignins and hemicelluloses, and cellulose from maize stems, rye straw, and rice straw. Polym. Degrad. Stab. 2001, 74, 307–319. [Google Scholar] [CrossRef]
- García, A.; González Alriols, M.; Spigno, G.; Labidi, J. Lignin as natural radical scavenger. Effect of the obtaining and purification processes on the antioxidant behaviour of lignin. Biochem. Eng. J. 2012, 67, 173–185. [Google Scholar] [CrossRef]
- Kim, I.; Han, J.-I. Optimization of alkaline pretreatment conditions for enhancing glucose yield of rice straw by response surface methodology. Biomass Bioenergy 2012, 46, 210–217. [Google Scholar] [CrossRef]
- Rawat, R.; Kumbhar, B.K.; Tewari, L. Optimization of alkali pretreatment for bioconversion of poplar (Populus deltoides) biomass into fermentable sugars using response surface methodology. Ind. Crops Prod. 2013, 44, 220–226. [Google Scholar] [CrossRef]
- Cotana, F.; Barbanera, M.; Foschini, D.; Lascaro, E.; Buratti, C. Preliminary Optimization of Alkaline Pretreatment for Ethanol Production from Vineyard Pruning. Energy Procedia 2015, 82, 389–394. [Google Scholar] [CrossRef] [Green Version]
- Łukajtis, R.; Rybarczyk, P.; Kucharska, K.; Konopacka-Łyskawa, D.; Słupek, E.; Wychodnik, K.; Kamiński, M. Optimization of saccharification conditions of lignocellulosic biomass under alkaline pre-treatment and enzymatic hydrolysis. Energies 2018, 11, 886. [Google Scholar] [CrossRef] [Green Version]
- Sun, R.-C.; Sun, X.-F.; Zhang, S.-H. Quantitative determination of hydroxycinnamic acids in wheat, rice, rye, and barley straws, maize stems, oil palm frond fiber, and fast-growing poplar wood. J. Agric. Food Chem. 2001, 49, 5122–5129. [Google Scholar] [CrossRef] [PubMed]
- Buranov, A.U.; Mazza, G. Extraction and purification of ferulic acid from flax shives, wheat and corn bran by alkaline hydrolysis and pressurised solvents. Food Chem. 2009, 115, 1542–1548. [Google Scholar] [CrossRef]
- Brienzo, M.; Siqueira, A.F.; Milagres, A.M.F. Search for optimum conditions of sugarcane bagasse hemicellulose extraction. Biochem. Eng. J. 2009, 46, 199–204. [Google Scholar] [CrossRef]
- Hernández-Salas, J.M.; Villa-Ramírez, M.S.; Veloz-Rendón, J.S.; Rivera-Hernández, K.N.; González-César, R.A.; Plascencia-Espinosa, M.A.; Trejo-Estrada, S.R. Comparative hydrolysis and fermentation of sugarcane and agave bagasse. Bioresour. Technol. 2009, 100, 1238–1245. [Google Scholar] [CrossRef]
- Saha, B.C.; Iten, L.B.; Cotta, M.A.; Wu, Y.V. Dilute acid pretreatment, enzymatic saccharification, and fermentation of rice hulls to ethanol. Biotechnol. Prog. 2005, 21, 816–822. [Google Scholar] [CrossRef]
- Paripati, P.; Dadi, A.P. Pretreatment and Fractionation of Lignocellulosic Biomass [Internet]. U.S. Patent 20140273104 A1, 18 September 2014. Available online: http://www.google.com/patents/US20140273104 (accessed on 3 December 2015).
- Sixta, H.; Schild, G. A new generation Kraft process. Lenzing. Ber. 2009, 87, 26–37. [Google Scholar]
- Sánchez, Ó.J.; Cardona, C.A. Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour. Technol. 2008, 99, 5270–5295. [Google Scholar] [CrossRef]
- Sarkanen, S.; Teller, D.C.; Stevens, C.R.; McCarthy, J.L. Associative interactions between Kraft lignin components. Macromolecules 1984, 17, 2588–2597. [Google Scholar] [CrossRef]
- Shi, H.; Fatehi, P.; Xiao, H.; Ni, Y. A combined acidification/PEO flocculation process to improve the lignin removal from the pre-hydrolysis liquor of kraft-based dissolving pulp production process. Bioresour. Technol. 2011, 102, 5177–5182. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Chen, H. Fractionation of alkali-extracted lignin from steam-exploded stalk by gradient acid precipitation. Sep. Purif. Technol. 2013, 105, 98–105. [Google Scholar] [CrossRef]
- Alekhina, M.; Ershova, O.; Ebert, A.; Heikkinen, S.; Sixta, H. Softwood kraft lignin for value-added applications: Fractionation and structural characterization. Ind. Crops Prod. 2015, 66, 220–228. [Google Scholar] [CrossRef]
- Axegård, P.; Öhman, F.; Theliander, H.; Tomani, P. Method for Separating Lignin from Black Liquor [Internet]. WO 2006031175 A1, 23 March 2006. Available online: http://www.google.fr/patents/WO2006031175A1 (accessed on 4 September 2015).
- Littorin, A.; Wallmo, H.; Norberg, H.; Wimby, M. Method for Precipitating Lignin from Black Liquor by Utilizing Waste Gases [Internet]. WO 2010143997 A1, 16 December 2010. Available online: http://www.google.com/patents/WO2010143997A1 (accessed on 4 September 2015).
- Öhman, F.; Theliander, H.; Norgren, M.; Tomani, P.; Axegård, P. Method for Separating Lignin from A Lignin Containing Liquid/Slurry [Internet]. WO 2006038863 A1, 13 April 2006. Available online: http://www.google.fr/patents/WO2006038863A1 (accessed on 4 September 2015).
- Wallmo, H.; Wimby, M. Method for lignin Separation from Black Liquor Having Removal of Sulphur Compounds from Formed Water Effluent [Internet]. U.S. Patent 20140357847 A1, 4 December 2014. Available online: http://www.google.fr/patents/US20140357847 (accessed on 4 September 2015).
- Minu, K.; Jiby, K.K.; Kishore, V.V.N. Isolation and purification of lignin and silica from the black liquor generated during the production of bioethanol from rice straw. Biomass Bioenergy 2012, 39, 210–217. [Google Scholar] [CrossRef]
- Glasser, W.G.; Wright, R.S. Steam-assisted biomass fractionation. II. fractionation behavior of various biomass resources. Biomass Bioenergy 1998, 14, 219–235. [Google Scholar] [CrossRef]
- Mousavioun, P.; Doherty, W.O.S. Chemical and thermal properties of fractionated bagasse soda lignin. Ind. Crops Prod. 2010, 31, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Gilarranz, M.A.; Rodriguez, F.; Oliet, M.; Revenga, J.A. Acid precipitation and purification of wheat straw lignin. Sep. Sci. Technol. 1998, 33, 1359–1377. [Google Scholar] [CrossRef]
- Umemura, M.; Yuguchi, Y. Solvation of xyloglucan in water/alcohol systems by molecular dynamics simulation. Cellulose 2009, 16, 361–371. [Google Scholar] [CrossRef]
- Sedlmeyer, F.B. Xylan as by-product of biorefineries: Characteristics and potential use for food applications. Food Hydrocoll. 2011, 25, 1891–1898. [Google Scholar] [CrossRef]
- Xu, J.; Yue, R.-Q.; Liu, J.; Ho, H.-M.; Yi, T.; Chen, H.-B.; Han, Q.B. Structural diversity requires individual optimization of ethanol concentration in polysaccharide precipitation. Int. J. Biol. Macromol. 2014, 67, 205–209. [Google Scholar] [CrossRef] [Green Version]
- Brillouet, J.-M.; Joseleau, J.-P.; Utille, J.-P.; Lelièvre, D. Isolation, purification and characterization of a complex heteroxylan from industrial wheat bran. J. Agric. Food Chem. 1982, 30, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Bian, J.; Peng, F.; Peng, P.; Xu, F.; Sun, R.-C. Isolation and fractionation of hemicelluloses by graded ethanol precipitation from Caragana korshinskii. Carbohydr. Res. 2010, 345, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Peng, F.; Bian, J.; Xu, F.; Sun, R. Studies on the starch and hemicelluloses fractionated by graded ethanol precipitation from bamboo Phyllostachys bambusoides f. shouzhu Yi. J. Agric. Food Chem. 2011, 59, 2680–2688. [Google Scholar] [CrossRef] [PubMed]
- Zeitoun, R.; Pontalier, P.Y.; Marechal, P.; Rigal, L. Twin-screw extrusion for hemicellulose recovery: Influence on extract purity and purification performance. Bioresour. Technol. 2010, 101, 9348–9354. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Du, R.; Guo, H.; Cao, M.; Wu, Q.; Su, R.; Qi, W.; He, Z. Fractional pretreatment of lignocellulose by alkaline hydrogen peroxide: Characterization of its major components. Food Bioprod. Process. 2015, 94, 322–330. [Google Scholar] [CrossRef]
- Sun, R.; Tomkinson, J. Fractional separation and physico-chemical analysis of lignins from the black liquor of oil palm trunk fibre pulping. Sep. Purif. Technol. 2001, 24, 529–539. [Google Scholar] [CrossRef]
- Zhao, J.; Ou, S.; Ding, S.; Wang, Y.; Wang, Y. Effect of activated charcoal treatment of alkaline hydrolysates from sugarcane bagasse on purification of p-coumaric acid. Chem. Eng. Res. Des. 2011, 89, 2176–2181. [Google Scholar] [CrossRef]
- Shen, J.; Kaur, I.; Baktash, M.M.; He, Z.; Ni, Y. A combined process of activated carbon adsorption, ion exchange resin treatment and membrane concentration for recovery of dissolved organics in pre-hydrolysis liquor of the kraft-based dissolving pulp production process. Bioresour. Technol. 2013, 127, 59–65. [Google Scholar] [CrossRef]
- Andersson, K.I.; Eriksson, M.; Norgren, M. Removal of lignin from wastewater generated by mechanical pulping using activated charcoal and fly ash: Adsorption isotherms and thermodynamics. Ind. Eng. Chem. Res. 2011, 50, 7722–7732. [Google Scholar] [CrossRef]
- Jung, M.-W.; Ahn, K.-H.; Lee, Y.; Kim, K.-P.; Rhee, J.-S.; Tae Park, J.; Paeng, K.J. Adsorption characteristics of phenol and chlorophenols on granular activated carbons (GAC). Microchem. J. 2001, 70, 123–131. [Google Scholar] [CrossRef]
- Caetano, M.; Valderrama, C.; Farran, A.; Cortina, J.L. Phenol removal from aqueous solution by adsorption and ion exchange mechanisms onto polymeric resins. J. Colloid Interface Sci. 2009, 338, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Alén, R.; Sjöström, E.; Suominen, S. Application of ion-exclusion chromatography to alkaline pulping liquors; separation of hydroxy carboxylic acids from inorganic solids. J. Chem. Technol. Biotechnol. 1991, 51, 225–233. [Google Scholar] [CrossRef]
- Abels, C.; Carstensen, F.; Wessling, M. Membrane processes in biorefinery applications. J. Membr. Sci. 2013, 444, 285–317. [Google Scholar] [CrossRef]
- Haddad, M.; Bazinet, L.; Savadogo, O.; Paris, J. A feasibility study of a novel electro-membrane based process to acidify Kraft black liquor and extract lignin. Process Saf. Environ. Prot. 2017, 106, 68–75. [Google Scholar] [CrossRef]
- Huang, H.-J.; Ramaswamy, S.; Tschirner, U.W.; Ramarao, B.V. A review of separation technologies in current and future biorefineries. Sep. Purif. Technol. 2008, 62, 1–21. [Google Scholar] [CrossRef]
- Persson, T.; Jönsson, A.-S. Isolation of hemicelluloses by ultrafiltration of thermomechanical pulp mill process water—Influence of operating conditions. Chem. Eng. Res. Des. 2010, 88, 1548–1554. [Google Scholar] [CrossRef]
- Toledano, A.; Serrano, L.; Garcia, A.; Mondragon, I.; Labidi, J. Comparative study of lignin fractionation by ultrafiltration and selective precipitation. Chem. Eng. J. 2010, 157, 93–99. [Google Scholar] [CrossRef]
- Wallberg, O.; Jönsson, A.-S. Separation of lignin in kraft cooking liquor from a continuous digester by ultrafiltration at temperatures above 100 °C. Desalination 2006, 195, 187–200. [Google Scholar] [CrossRef]
- Jönsson, A.-S.; Wallberg, O. Cost estimates of kraft lignin recovery by ultrafiltration. Desalination 2009, 237, 254–267. [Google Scholar] [CrossRef]
- Liu, G.; Liu, Y.; Shi, H.; Qian, Y. Application of inorganic membranes in the alkali recovery process. Desalination 2004, 169, 193–205. [Google Scholar] [CrossRef]
- Singh, S.C.; Murthy, Z.V.P. Hemicelluloses separation from caustic-containing process stream by ultrafiltration. Sep. Sci. Technol. 2017, 52, 2252–2261. [Google Scholar] [CrossRef]
- Oriez, V.; Peydecastaing, J.; Pontalier, P.-Y. Separation of sugarcane bagasse mild alkaline extract components by ultrafiltration—Membrane screening and effect of filtration parameters. Process Biochem. 2019, 78, 91–99. [Google Scholar] [CrossRef] [Green Version]
- Ajao, O.; Rahni, M.; Marinova, M.; Chadjaa, H.; Savadogo, O. Retention and flux characteristics of nanofiltration membranes during hemicellulose prehydrolysate concentration. Chem. Eng. J. 2015, 260, 605–615. [Google Scholar] [CrossRef]
- Lucas, J.L.; Martin, F.E. Process for Production of Lignin Fuel, Ethyl Alcohol, Cellulose, Silica/Silicates, and Cellulose Derivatives from Plant Biomass [Internet]. U.S. Patent 5735916A, 7 April 1998. Available online: http://www.google.com/patents/US5735916 (accessed on 20 May 2016).
- Nilsson, M.; Trägårdh, G.; Östergren, K. Influence of temperature and cleaning on aromatic and semi-aromatic polyamide thin-film composite NF and RO membranes. Sep. Purif. Technol. 2008, 62, 717–726. [Google Scholar] [CrossRef]

| Biomass | Variable | Optimized Conditions | Solubilization Yields | Reference |
|---|---|---|---|---|
| SCB (dewaxed) | 1–3% NaOH (w/v) | 3% NaOH (w/v) | 54% lignin 75% hemicelluloses | [53] |
| No variation of S:L ratio | S:L ratio of 1:25 | |||
| No variation of temperature | 50 °C | |||
| No variation of duration | 3 h | |||
| SCB (dewaxed) | No variation of alkaline concentration | 2% NaOH (w/v) | 55% lignin 48% hemicelluloses | [54] |
| No variation of S:L ratio | S:L ratio of 1:30 | |||
| No variation of temperature | 55 °C | |||
| No variation of duration | 2 h | |||
| SCB | 2–6% NaOH (w/v) | 4% NaOH | 43% hemicelluloses | [55] |
| No variation of S:L ratio | S:L ratio 5:1 | |||
| 150–190 °C | 170 °C | |||
| 40–80 min | 1 h | |||
| SCB | No variation of alkaline concentration | 1.5% NaOH (w/v) | 46% lignin 22% xylan 50% arabinan | [30] |
| No variation of S:L ratio | S:L ratio of 1:20 (w/v) | |||
| No variation of temperature | 60 °C | |||
| No variation of duration | 6 h | |||
| SCB | No variation of alkaline concentration | 2% NaOH (w/v) | 70% lignin 22% xylan 22% arabinan | [29] |
| No variation of S:L ratio | S:L ratio 1:10 (w/v) | |||
| No variation of temperature | 80 °C | |||
| No variation of duration | 2 h | |||
| SCB | No variation of alkaline concentration | 2% Ca(OH)2 (w/v) | 28% lignin 9% xylan 6% arabinan | [29] |
| No variation of S:L ratio | S:L ratio 1:10 (w/v) | |||
| No variation of temperature | 80 °C | |||
| No variation of duration | 2 h | |||
| SCB | No variation of alkaline concentration | 1% Ca(OH)2 (w/v) | 14% lignin 0% hemicelluloses | [47] |
| No variation of S:L ratio | S:L ratio of 1:10 | |||
| No variation of temperature | 120 °C | |||
| No variation of duration | 1 h | |||
| SCB | No variation of alkaline concentration | 1% Ca(OH)2 (w/v) | 30% lignin 5% hemicelluloses | [48] |
| No variation of S:L ratio | S:L ratio of 1:100 | |||
| No variation of temperature | 120 °C | |||
| 7–60 min | 1 h | |||
| SCB | 0.03–0.3% NH3 (w/w) | 0.3% NH3 (w/w) | 46% lignin 27% hemicelluloses | [56] |
| No variation of S:L ratio | S:L ratio of 1:8 (w/v) | |||
| No variation of temperature | 30 °C | |||
| 0–40 day | 40 day | |||
| SCB | 20–28% NH3 (v/v) | 20% NH3 (v/v) | 42% lignin 69% hemicelluloses | [57] |
| No variation of S:L ratio | S:L ratio of 1:10 (w/v) | |||
| 50–70 °C | 70 °C | |||
| 8–24 h | 24 h | |||
| Wheat straw | 3% NaOH (w/v) | 3% NaOH (w/v) | 70% lignin | [37] |
| S:L ratio of 1:26.6 (w/v) | S:L ratio of 1:26.6 (w/v) | |||
| 45 °C | 45 °C | |||
| 2–15 h | 15 h | |||
| Wheat straw | 0.5-10% NaOH (w/v) | 1.5% NaOH (w/v) | 59% lignin 83% hemicelluloses | [34] |
| No variation of S:L ratio | S:L ratio 1:40 (w/v) | |||
| 0–80 °C | 20 °C | |||
| 0–144 h | 144 h | |||
| Wheat straw | 0.5–10% NaOH (w/v) | 1.5% NaOH (w/v) | 62% lignin 75% hemicelluloses | [34] |
| No variation of S:L ratio | S:L ratio 1:40 (w/v) | |||
| 0–80 °C | 80 °C | |||
| 0–144 h | 6 h | |||
| Wheat straw | 0.5–10% NaOH (w/v) | 10% NaOH (w/v) | 47% lignin 81% hemicelluloses | [34] |
| No variation of S:L ratio | S:L ratio 1:40 (w/v) | |||
| 0–80 °C | 20 °C | |||
| 0–144 h | 6 h | |||
| Maize stems | No variation of alkaline concentration | 4% NaOH (w/v) | 78% lignin 72% hemicelluloses | [58] |
| No variation of S:L ratio | S:L ratio of 1:100 | |||
| No variation of temperature | 30 °C | |||
| No variation of duration | 18 h | |||
| Rye straw | No variation of alkaline concentration | 4% NaOH (w/v) | 79% lignin 73% hemicelluloses | [58] |
| No variation of S:L ratio | S:L ratio of 1:100 | |||
| No variation of temperature | 30 °C | |||
| No variation of duration | 18 h | |||
| Rice straw | No variation of alkaline concentration | 4% NaOH (w/v) | 82% lignin 85% hemicelluloses | [58] |
| No variation of S:L ratio | S:L ratio of 1:100 | |||
| No variation of temperature | 30 °C | |||
| No variation of duration | 18 h | |||
| Sorghum bagasse | 2–10% NaOH (w/v) | 4% NaOH (w/v) | 76% lignin 60% xylan | [32] |
| No variation of S:L ratio | S:L ratio of 1:20 (w/v) | |||
| No variation of temperature | 25 °C | |||
| 0.5–2 h | 2 h | |||
| Sorghum bagasse | 2–10% NaOH (w/v) | 10% NaOH (w/v) | 80% lignin 81% xylan | [32] |
| No variation of S:L ratio | S:L ratio of 1:20 (w/v) | |||
| No variation of temperature | 25 °C | |||
| 0.5–2 h | 2 h |
| Purification Technologies | Conditions | Mode of Action | Limits |
|---|---|---|---|
| Flocculation | Addition of divalent cation (e.g., Ca2+, Mg2+) Batch process | Flocculation of lignin | Limited to sodium hydroxide extracts, low recovery of lignin |
| Precipitation by acid addition | Sulfuric acid is mainly used (2% v/v to 72% v/v) pH adjusted to 2–5 Temperature: 60–85 °C Batch process | Precipitation of lignin (80–95%) | Lignin purity: co-precipitation of sugars, extensive washing of the precipitated lignin to remove the salts |
| Precipitation by ethanol addition | Final ethanol concentration in the hydrolysate: 80–95% v/v Room temperature Batch process | Precipitation of hemicelluloses (80–95%) | Hemicelluloses purity: co-precipitation of lignin High volume of solvent is required (recycling is necessary) |
| Adsorption on AC | AC:hydrolysate ratio 3:100 (w/v) Batch process | Adsorption of lignin and ferulic acid (80–98%) Low adsorption of hemicelluloses and p-CA | Sodium hydroxide 2% (w/v) is required for the desorption |
| Adsorption on non-functionalized resin | pH adjustment (acidification) Resin packed in a column Continuous process can potentially be applied | Adsorption of phenolic compounds (about 60%) | Low adsorption capacity Desorption with organic solvent |
| Adsorption on anion-exchange resin | No pH adjustment Resin packed in a column Continuous process can potentially be applied | Adsorption of phenolic compounds (70–90%) Desorption up to 99% (e.g., for p-CA) | Desorption with sodium chloride (4%) or organic solvent |
| Low-pressure chromatography | Macroporous cation-exchange resin Water as eluent Continuous process can potentially be applied | High molecular weight lignin and hemicelluloses obtained in a fast eluted fraction free of salts, phenolic monomers, and acetic acid | Low lignin and hemicelluloses recovery (15% and 30%, respectively) |
| Membrane filtration | High temperatures (about 100 °C) on ceramic membranes leading to high fluxes Lower temperatures (20–40 °C) on organic membranes UF and NF membranes (MWCO from 100 Da to 30 kDa) Continuous process can potentially be applied | Lignin and hemicelluloses recovered in the retentate to different extent (up to 95%) Phenolic monomers, acetic acid, salts collected in the permeate | Usually no separation of lignin and hemicelluloses (but it depends on the biomass and the alkaline conditions of the fractionation) |
| Electrodialysis | Current density: 330 A/m2 Temperature: 35 °C Batch process so far | Precipitation of lignin via acidification and recovery of sodium ions | Studies are missing |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oriez, V.; Peydecastaing, J.; Pontalier, P.-Y. Lignocellulosic Biomass Mild Alkaline Fractionation and Resulting Extract Purification Processes: Conditions, Yields, and Purities. Clean Technol. 2020, 2, 91-115. https://doi.org/10.3390/cleantechnol2010007
Oriez V, Peydecastaing J, Pontalier P-Y. Lignocellulosic Biomass Mild Alkaline Fractionation and Resulting Extract Purification Processes: Conditions, Yields, and Purities. Clean Technologies. 2020; 2(1):91-115. https://doi.org/10.3390/cleantechnol2010007
Chicago/Turabian StyleOriez, Vincent, Jérôme Peydecastaing, and Pierre-Yves Pontalier. 2020. "Lignocellulosic Biomass Mild Alkaline Fractionation and Resulting Extract Purification Processes: Conditions, Yields, and Purities" Clean Technologies 2, no. 1: 91-115. https://doi.org/10.3390/cleantechnol2010007