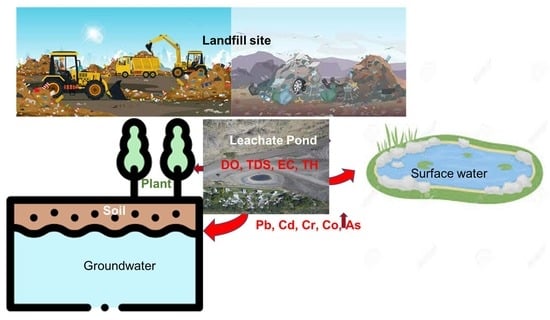
Impacts of Landfill Leachate on the Surrounding Environment: A Case Study on Amin Bazar Landfill, Dhaka (Bangladesh)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection
2.3. Sample Preparation
2.4. Sample Analysis
2.5. Statistical Analysis and Spatial Distribution of Metal(loid)s
2.6. Indices for Water Quality, Pollution, and Feasibility Assessment
3. Results and Discussion
3.1. Physicochemical Characterization of Leachate and Water Samples
3.2. Concentration of Metal(loid)s in Leachate, Water, Soil, and Plants Samples
3.3. Principal Component Analysis
3.4. Pearson’s Correlation Analysis
3.5. Water Quality and Pollution Assessment
3.6. Spatial Comparison of Heavy Metal(loid)s Concentration and Distance from Landfill Site
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Christensen, T.H.; Kjeldsen, P. Basic biochemical processes in landfills. In Sanitary Landfilling: Process, Technology and Environmental Impact; Christensen, T.C., Ed.; Academic Press: London, UK, 1989; p. 29. [Google Scholar]
- Kamal, A.K.I.; Islam, R.; Hassan, M.; Ahmed, F.; Rahman, M.A.T.M.T.; Moniruzzaman, M. Bioaccumulation of Trace Metals in Selected Plants within Amin Bazar Landfill Site, Dhaka, Bangladesh. Environ. Process. 2016, 3, 179–194. [Google Scholar] [CrossRef]
- Mukti, S.A. Solid Waste Management in Dhaka City: Problems and Prospects. Int. J. Innov. Res. Dev. 2015, 2, 33–37. [Google Scholar]
- DNCC. Waste Report; Dhaka North City Corporation: Dhaka, Bangladesh, 2020. [Google Scholar]
- Hoque, M.A.; Haque, M.A.; Mondal, M.S.A. Seasonal Effects on Heavy Metal Concentration in Decomposed Solid Waste of DNCC and DSCC Landfill Sites. Civ. Eng. Arch. 2014, 2, 52–56. [Google Scholar] [CrossRef]
- Kabir, M.R. Municipal solid waste management system: A study on Dhaka north and South City corporations. Bangladesh Inst. Plan 2015, 2075, 9363. [Google Scholar]
- Yousuf, T.B.; Rahman, M. Monitoring quantity and characteristics of municipal solid waste in Dhaka City. Environ. Monit. Assess 2007, 135, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Azim, M.D.; Rahman, M.M.; Khan, R.H.; Kamal, A. Characteristics of Leachate Generated at Landfill Sites and Probable Risks of Surface and Groundwater Pollution in The Surrounding Areas: A Case Study of Matuail Landfill Site, Dhaka. J. Bangladesh Acad. Sci. 1970, 35, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Gavrilescu, M.; Schiopu, A.-M.; Robu, B.M.; Apostol, I. Impact of landfill leachate on soil quality in Iasi county. Environ. Eng. Manag. J. 2009, 8, 1155–1164. [Google Scholar] [CrossRef]
- Negi, P.; Mor, S.; Ravindra, K. Impact of landfill leachate on the groundwater quality in three cities of North India and health risk assessment. Environ. Dev. Sustain. 2018, 22, 1455–1474. [Google Scholar] [CrossRef]
- Jahan, E.; Nessa, A.; Hossain, F.; Parveen, Z. Characteristics of municipal landfill leachate and its impact on surrounding agricultural land. Bangladesh J. Sci. Res. 2016, 29, 31–39. [Google Scholar] [CrossRef] [Green Version]
- El-Salam, M.M.A.; Abu-Zuid, G.I. Impact of landfill leachate on the groundwater quality: A case study in Egypt. J. Adv. Res. 2014, 6, 579–586. [Google Scholar] [CrossRef]
- Naveen, B.; Mahapatra, D.M.; Sitharam, T.; Sivapullaiah, P.; Ramachandra, T. Physico-chemical and biological characterization of urban municipal landfill leachate. Environ. Pollut. 2017, 220, 1–12. [Google Scholar] [CrossRef]
- Adhikari, B.; Khanal, S.N.; Manandhar, D.R. Study of leachate and waste composition at different landfill sites of Nepal. Kathmandu Univ. J. Sci. Eng. Technol. 2013, 9, 15–21. [Google Scholar]
- Chang, A.C.; Page, A.L. Trace elements slowly accumulating, depleting in soils. Calif. Agric. 2000, 54, 49–55. [Google Scholar] [CrossRef]
- Alam, H. Second Modern Sanitary Landfill at Amin Bazar Awaits Green Signal. The Daily Star. 2008. Available online: http://archive.thedailystar.net/newDesign/news-details.php?nid=52541 (accessed on 20 December 2021).
- Ahsan, A.; Satter, F.; Siddique, A.B.; Akbor, A.; Ahmed, S.; Shajahan, M.; Khan, R. Chemical and physicochemical characterization of effluents from the tanning and textile industries in Bangladesh with multivariate statistical approach. Environ. Monit. Assess. 2019, 191, 575. [Google Scholar] [CrossRef]
- Siddique, A.B.; Alam, K.; Islam, S.; Diganta, M.T.M.; Akbor, A.; Bithi, U.H.; Chowdhury, A.I.; Ullah, A.K.M.A. Apportionment of some chemical elements in soils around the coal mining area in northern Bangladesh and associated health risk assessment. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100366. [Google Scholar] [CrossRef]
- Hasan, A.B.; Reza, A.H.M.S.; Kabir, S.; Siddique, A.B.; Ahsan, A.; Akbor, A. Accumulation and distribution of heavy metals in soil and food crops around the ship breaking area in southern Bangladesh and associated health risk assessment. SN Appl. Sci. 2020, 2, 155. [Google Scholar] [CrossRef] [Green Version]
- Nasrin, S.; Islam, M.N.; Abu Tayab, M.; Nasrin, M.S.; Siddique, A.B.; Bin Emran, T.; Reza, A.A. Chemical profiles and pharmacological insights of Anisomeles indica Kuntze: An experimental chemico-biological interaction. Biomed. Pharmacother. 2022, 149, 112842. [Google Scholar] [CrossRef]
- APHA (American Public Health Association). Standard Methods for the Examination of Water and Wastewater, 23rd ed.; Baird, R.B., Eaton, A.D., Rice, E.W., Eds.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2017; Available online: https://secure.apha.org/imis/ItemDetail?iProductCode=978-087553-2875&CATEGORY=BK (accessed on 20 December 2021).
- Childs, C. Interpolating surfaces in ArcGIS spatial analyst. ArcUser 2004, 3235, 32–35. [Google Scholar]
- Gao, B.; Gao, L.; Gao, J.; Xu, D.; Wang, Q.; Sun, K. Simultaneous evaluations of occurrence and probabilistic human health risk associated with trace elements in typical drinking water sources from major river basins in China. Sci. Total Environ. 2019, 666, 139–146. [Google Scholar] [CrossRef]
- Rahman, M.A.T.M.T.; Paul, M.; Bhoumik, N.; Hassan, M.; Alam, K.; Aktar, Z. Heavy metal pollution assessment in the groundwater of the Meghna Ghat industrial area, Bangladesh, by using water pollution indices approach. Appl. Water Sci. 2020, 10, 186. [Google Scholar] [CrossRef]
- Abrahim, G.M.S.; Parker, R.J. Assessment of heavy metal enrichment factors and the degree of contamination in marine sediments from Tamaki Estuary, Auckland, New Zealand. Environ. Monit. Assess. 2007, 136, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Sharmin, S.; Mia, J.; Miah, M.S.; Zakir, H. Hydrogeochemistry and heavy metal contamination in groundwaters of Dhaka metropolitan city, Bangladesh: Assessment of human health impact. J. Hydro-Environ. Res. 2020, 3, 106–117. [Google Scholar] [CrossRef]
- Doza, B.; Islam, S.D.-U.; Rume, T.; Quraishi, S.B.; Rahman, M.S.; Bhuiyan, M.A.H. Groundwater quality and human health risk assessment for safe and sustainable water supply of Dhaka City dwellers in Bangladesh. Groundw. Sustain. Dev. 2020, 10, 100374. [Google Scholar] [CrossRef]
- Islam, A.R.M.T.; Islam, H.T.; Mia, U.; Khan, R.; Habib, A.; Doza, B.; Siddique, A.B.; Chu, R. Co-distribution, possible origins, status and potential health risk of trace elements in surface water sources from six major river basins, Bangladesh. Chemosphere 2020, 249, 126180. [Google Scholar] [CrossRef] [PubMed]
- Backman, B.; Bodiš, D.; Lahermo, P.; Rapant, S.; Tarvainen, T. Application of a groundwater contamination index in Finland and Slovakia. Environ. Geol. 1998, 36, 55–64. [Google Scholar] [CrossRef]
- Edet, A.E.; Offiong, O.E. Evaluation of water quality pollution indices for heavy metal contamination monitoring. A study case from Akpabuyo-Odukpani area, Lower Cross River Basin (Southeastern Nigeria). GeoJournal 2002, 57, 295–304. [Google Scholar] [CrossRef]
- Horton, R.K. An index system for rating water quality. J. Water Pollut. Control Ed. 1965, 37, 300–306. [Google Scholar]
- Mohan, S.V.; Nithila, P.; Reddy, S.J. Estimation of heavy metals in drinking water and development of heavy metal pollution index. J. Environ. Sci. Health Part A Environ. Sci. Eng. Toxicol. 1996, 31, 283–289. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, L.; Deng, L.; Jin, Z. Characteristics, sources, water quality and health risk assessment of trace elements in river water and well water in the Chinese Loess Plateau. Sci. Total. Environ. 2018, 650, 2004–2012. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Guidelines for Drinking Water Quality, Library Cataloguing-in-Publication Data, 4th ed.; NLM classification: WA 675; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- ECR (The Environment Conservation Rules); Government of the People’s Republic of Bangladesh, Ministry of Environment and Forest: Dhaka, Bangladesh, 1997.
- USEPA. US Environmental Protection Agency. Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual (part E, Supplemental Guidance for Dermal Risk Assessment) Final; EPA/540/R/99/005 OSWER 9285.702EP PB99-963312 July 2004; Office of Super fund Remediation and Technology Innovation: Washington, DC, USA, 2004. [Google Scholar]
- WHO (World Health Organization). Guidelines for Drinking Water Quality, Library Cataloguing in Publication Data, 3rd ed.; NLM classification: WA 675; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- World Health Organization. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; World Health Organization: Geneva, Switzerland, 2017; ISBN 9789241549950. [Google Scholar]
- Tchobanoglous, G.; Theisen, H.; Vigil, S.A. Integrated Solid Waste Management: Engineering Principles and Management Issues; McGraw-Hill: New York, NY, USA, 1993. [Google Scholar] [CrossRef]
- Kumar, D.; Alappat, B.J. Analysis of leachate pollution index and formulation of sub-leachate pollution indices. Waste Manag. Res. 2005, 23, 230–239. [Google Scholar] [CrossRef]
- Parvin, F.; Tareq, S.M. Impact of landfill leachate contamination on surface and groundwater of Bangladesh: A systematic review and possible public health risks assessment. Appl. Water Sci. 2021, 11, 100. [Google Scholar] [CrossRef]
- Alam, R.; Ahmed, Z.; Howladar, M.F. Evaluation of heavy metal contamination in water, soil and plant around the open landfill site Mogla Bazar in Sylhet, Bangladesh. Groundw. Sustain. Dev. 2019, 10, 100311. [Google Scholar] [CrossRef]
- Raviraja, A.; Babu, G.; Bijoor, A.; Menezes, G.; Venkatesh, T. Lead Toxicity in a Family as a Result of Occupational Exposure. Arch. Ind. Hyg. Toxicol. 2008, 59, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Riess, M.L.; Halm, J.K. Lead poisoning in an adult: Lead mobilization by pregnancy? J. Gen. Intern. Med. 2007, 22, 1212–1215. [Google Scholar] [CrossRef]
- Prasad, S.; Yadav, K.K.; Kumar, S.; Gupta, N.; Cabral-Pinto, M.M.; Rezania, S.; Radwan, N.; Alam, J. Chromium contamination and effect on environmental health and its remediation: A sustainable approaches. J. Environ. Manag. 2021, 285, 112174. [Google Scholar] [CrossRef]
- Wakeel, A.; Xu, M. Chromium Morpho-Phytotoxicity. Plants 2020, 9, 564. [Google Scholar] [CrossRef]
- Hussein, M.; Yoneda, K.; Mohd-Zaki, Z.; Amir, A.; Othman, N. Heavy Metals in Leachate, Impacted Soils and Natural Soils of Different Landfills in Malaysia: An Alarming Threat. Chemosphere 2020, 128874. [Google Scholar] [CrossRef] [PubMed]
- Battistel, M.; Stolze, L.; Muniruzzaman, M.; Rolle, M. Arsenic release and transport during oxidative dissolution of spatially-distributed sulfide minerals. J. Hazard. Mater. 2020, 409, 124651. [Google Scholar] [CrossRef]
- Tandel, B.N.; Macwan, J.E.M.; Soni, C.K. Assessment of water quality index of small lake in south Gujarath region, India. In Proceedings of the ISEM-2011, Bangkok, Thailand, 23–24 December 2011. [Google Scholar]
- Siddique, A.B.; Islam, A.R.M.T.; Hossain, S.; Khan, R.; Akbor, A.; Hasanuzzaman; Sajid, W.M.; Mia, Y.; Mallick, J.; Rahman, M.S.; et al. Multivariate statistics and entropy theory for irrigation water quality and entropy-weighted index development in a subtropical urban river, Bangladesh. Environ. Sci. Pollut. Res. 2021, 29, 8577–8596. [Google Scholar] [CrossRef]








| Sample | Sample Type | Location | Details of Sample | |
|---|---|---|---|---|
| Latitude | Longitude | |||
| 01 | Untreated leachate | 23°47′51.842″ | 90°17′52.571″ | Collected from the leachate pond. |
| 02 | Treated leachate | 23°47′51.842″ | 90°17′52.576″ | Collected from the leachate pond. |
| 03-01 | Surface water | 23°47′39.361″ | 90°17′47.176″ | Collected from the open lake exposed to the landfill at a depth of 50 cm. |
| 03-02 | Surface water | 23°47′34.842″ | 90°18′01.633″ | Collected from a pond at a depth of 50 cm. |
| 03-03 | Surface water | 23°47′33.541″ | 90°18′13.691″ | Collected from a pond at a depth of 50 cm. |
| 04-01 | Groundwater | 23°47′39.947″ | 90°17′44.797″ | At a depth of 70 ft (21.3 m). |
| 04-02 | Groundwater | 23°47′33.844″ | 90°18′07.443″ | At a depth of 280 ft (85.3 m). |
| 04-03 | Groundwater | 23°47′42.151″ | 90°18′15.884″ | At a depth of 400 ft (121.9 m). |
| 05-01 | Soil | 23°47′39.947″ | 90°17′44.797″ | Collected from 10 cm beneath the surface. |
| 05-02 | Soil | 23°47′33.547″ | 90°18′13.691″ | Collected from 10 cm beneath the surface. |
| 05-03 | Soil | 23°47′42.153″ | 90°18′15.882″ | Collected from 10 cm beneath the surface. |
| 06-01 | Plant | 23°47′39.947″ | 90°17′44.797″ |
|
| 06-02 | Plant | 23°47′34.845″ | 90°18′01.631″ |
|
| 06-03 | Plant | 23°47′33.545″ | 90°18′13.694″ |
|
| MAC (µg/L) | CNi (µg/L) | Ii (µg/L) | Si (µg/L) | Wi (µg/L) | References | |
|---|---|---|---|---|---|---|
| Pb | 10 | 1.5 | 10 | 50 | 0.02 | [33,34] |
| Cd | 3 | 3 | 3 | 5 | 0.20 | [34,35] |
| Cr | 50 | 50 | 50 | 100 | 0.01 | [34,36] |
| Co | 1000 | 1000 | 50 | 100 | 0.01 | [34,36] |
| Ni | 70 | 20 | 20 | 70 | 0.01 | [33,37,38] |
| As | 10 | 10 | 10 | 50 | 0.02 | [33,35] |
| Sample ID | Sample Type | pH | DO (mg/L) | Turbidity (FNU) | Salinity (mg/L) | TDS (mg/L) | EC (µS/cm) | Temperature (°C) | TH (mg/L) |
|---|---|---|---|---|---|---|---|---|---|
| 01 | Leachate | 7.85 | 1.47 | 6.58 | 10.4 | 5847 | 11,694 | 33.87 | 2805.6 |
| 02 | Leachate | 7.85 | 1.26 | 3.19 | 19.9 | 2980 | 5960 | 32.38 | 1132.3 |
| Mean | 7.85 | 1.36 | 4.88 | 15.2 | 4413.5 | 8827 | 33.12 | 1968.9 | |
| SD | 0.00 | 0.15 | 2.40 | 6.72 | 2027.3 | 4054.6 | 1.05 | 836.7 | |
| 03-01 | SW | 7.85 | 15.5 | 0.37 | 19.7 | 385 | 770 | 31.38 | 501 |
| 03-02 | SW | 7.05 | 3.50 | 7.30 | 0.22 | 231 | 458 | 27.63 | 498 |
| 03-03 | SW | 7.11 | 4.20 | 3.40 | 0.17 | 182 | 370 | 27.62 | 492 |
| Mean | 7.33 | 7.73 | 3.69 | 6.70 | 266 | 532.7 | 28.87 | 497 | |
| SD | 0.44 | 5.49 | 3.47 | 11.3 | 105.9 | 210.2 | 2.16 | 3.74 | |
| 04-01 | GW | 7.38 | 15.3 | 0.14 | 1.01 | 149 | 297 | 30.85 | 330 |
| 04-02 | GW | 7.30 | 4.50 | 0.40 | 0.16 | 171 | 342 | 27.74 | 288 |
| 04-03 | GW | 7.08 | 4.60 | 2.10 | 0.31 | 326 | 653 | 27.82 | 316 |
| Mean | 7.25 | 8.13 | 0.88 | 0.49 | 215.3 | 430.7 | 28.80 | 311.5 | |
| SD | 0.15 | 5.06 | 1.06 | 0.45 | 96.47 | 193.9 | 1.77 | 17.6 | |
| ECR, 1997 [28] | 6.5–8.5 | 6 | 10 | - | 1000 | 350 | 20–30 | - | |
| WHO, 2017 [30] | 6.5–8.0 | 4–6 | 5 | - | 500 | 250 | - | - | |
| Parameters | pH | DO | Turbidity | Salinity | TDS | EC | Temperature | TH |
|---|---|---|---|---|---|---|---|---|
| pH | 1 | |||||||
| DO | 0.125 | 1 | ||||||
| Turbidity | −0.069 | −0.667 | 1 | |||||
| Salinity | 0.906 | 0.107 | −0.058 | 1 | ||||
| TDS | 0.672 | −0.479 | 0.520 | 0.473 | 1 | |||
| EC | 0.672 | −0.479 | 0.520 | 0.473 | 1 | 1 | ||
| Temperature | 0.922 | 0.089 | 0.100 | 0.753 | 0.797 | 0.797 | 1 | |
| TH | 0.613 | −0.437 | 0.583 | 0.393 | 0.980 | 0.980 | 0.756 | 1 |
| Parameters | Pb | Cd | Cr | Co | Ni | As |
|---|---|---|---|---|---|---|
| Pb | 1 | |||||
| Cd | 0.533 | 1 | ||||
| Cr | 0.858 | 0.146 | 1 | |||
| Co | 0.831 | 0.188 | 0.823 | 1 | ||
| Ni | 0.861 | 0.180 | 0.854 | 0.995 | 1 | |
| As | 0.830 | 0.178 | 0.822 | 0.978 | 0.983 | 1 |
| Sample ID | Sample Types | WQI | CD | HEI | HPI |
|---|---|---|---|---|---|
| 01 | Leachate untreated | 50.01 | 3.317 | 9.317 | 16.445 |
| 02 | Leachate treated | 28.41 | 4.663 | 10.663 | −5.159 |
| Mean | 39.21 | 3.990 | 9.990 | 5.643 | |
| 03-01 | SW | 43.63 | −2.061 | 3.938 | 10.059 |
| 03-02 | SW | 25.33 | 0.162 | 6.162 | −8.232 |
| 03-03 | SW | 17.82 | −4.075 | 1.924 | −15.750 |
| Mean | 23.56 | −2.204 | 3.795 | −10.002 | |
| 04-01 | GW | 29.15 | −2.966 | 3.033 | −4.421 |
| 04-02 | GW | 31.41 | −3.878 | 2.121 | −2.158 |
| 04-03 | GW | 33.25 | −3.980 | 2.019 | −0.312 |
| Mean | 21.89 | −4.008 | 1.991 | −11.677 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hredoy, R.H.; Siddique, M.A.B.; Akbor, M.A.; Shaikh, M.A.A.; Rahman, M.M. Impacts of Landfill Leachate on the Surrounding Environment: A Case Study on Amin Bazar Landfill, Dhaka (Bangladesh). Soil Syst. 2022, 6, 90. https://doi.org/10.3390/soilsystems6040090
Hredoy RH, Siddique MAB, Akbor MA, Shaikh MAA, Rahman MM. Impacts of Landfill Leachate on the Surrounding Environment: A Case Study on Amin Bazar Landfill, Dhaka (Bangladesh). Soil Systems. 2022; 6(4):90. https://doi.org/10.3390/soilsystems6040090
Chicago/Turabian StyleHredoy, Rysul Hasan, Md. Abu Bakar Siddique, Md. Ahedul Akbor, Md. Aftab Ali Shaikh, and Md. Mostafizur Rahman. 2022. "Impacts of Landfill Leachate on the Surrounding Environment: A Case Study on Amin Bazar Landfill, Dhaka (Bangladesh)" Soil Systems 6, no. 4: 90. https://doi.org/10.3390/soilsystems6040090