1. Introduction
Fortunately, large numbers of individuals have now recovered from the acute coronavirus disease-19 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Unfortunately, there are growing numbers and concerns regarding a subset of these recovered individuals who have varied, persistent, and reoccurring symptoms relating to this pandemic that have termed themselves as ‘long haulers’, while the medical community has now termed this condition as long-haul COVID—long COVID (LC) or post-acute sequela of SARS-CoV-2 (PASC) [
1,
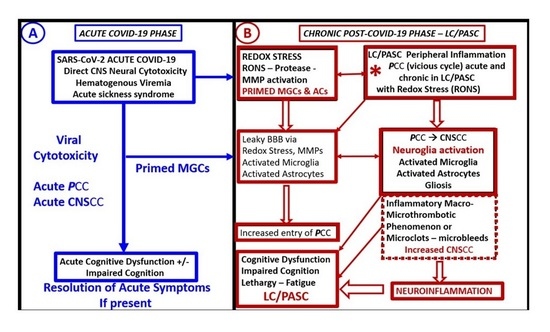
2]. Hereafter, the terms LC and PASC will be referred to as LC/PASC. It is important to note at this time that there is still not a standardized definition of LC/PASC; however, in the broadest sense, these individuals represent a failure to return to a baseline state of health after recovering from acute COVID-19 and encompass various physical, neurologic, and neuropsychiatric symptoms lasting from weeks to months following the acute infection of COVID-19. Typical symptoms of LC/PASC include fatigue (central/cognitive, or mental and peripheral/muscular or physical), shortness of breath, chest pain, “brain fog” (impaired cognition with difficulty in maintaining focus), sleep disorders (insomnia), fevers, gastrointestinal symptoms (diarrhea, nausea and/or vomiting), anxiety, and depression that can persist from weeks to months and can range from mild to incapacitating (
Figure 1) [
1,
2].
The NIH director, Francis Collins, has recently reported on the findings from a NIH workshop entitled “Workshop on Post-Acute Sequelae of SARS-CoV-2 (PASC)”, in which he summarized what is known about post-COVID-19 symptoms and identified key gaps in the knowledge surrounding this [
3]. In addition to this workshop, it was announced previously in December 2020 that the United States Congress has provided 1.15 billion dollars in funding to be made available over four years for the NIH to support research into the prolonged health consequences of SARS-CoV-2 infection. Some of the key questions in regards to this funding include: (1) “What does the spectrum of recovery from SARS-CoV-2 infection look like across the population?”; (2) “How many people continue to have symptoms of COVID-19, or even develop new symptoms, after acute SARS-CoV-2 infection?”; (3) “How many people continue to have symptoms of COVID-19, or even develop new symptoms, after acute SARS-CoV-2 infection?”; (4) “What is the underlying biological cause of these prolonged symptoms?”; (5) “What makes some people vulnerable to this but not others?”; and (6) “Does SARS-CoV-2 infection trigger changes in the body that increase the risk of other conditions, such as chronic heart or brain disorders?” [
3]. It is anticipated that these questions might stimulate further research to investigate opportunities that will focus on clinical trials to test treatment strategies regarding PASC symptoms and, thus, promote recovery [
3]. Additionally, there exists the need to access the emotional, behavioral, and societal impact of the COVID-19 pandemic across all nations [
4].
Specific time frames of LC/PASC have not been universally defined at the time of this manuscript’s preparation; however, there seems to be an emerging consensus of greater than four weeks post-acute COVID-19 [
5]. However, authors speculate that the time frame may ultimately be between four to eight weeks and that this time frame might be more in line with the normal healing time as occurs in the response to injury wound healing mechanisms that are at play in response to non-central nervous system tissue/organ injuries [
6,
7,
8,
9,
10,
11]. The central nervous system (CNS) is unique in its response to injury wound healing mechanisms since the brain does not have fibrocytes/fibroblasts as in peripheral tissues, which is important for fibrosis and scar formation; however, the brain possesses neuroglial astrocyte(s) (ACs) that are capable of undergoing activation and reactive astrogliosis that is responsible for the protective glial scar formation in the brain following injury (
Figure 2) [
10,
11].
Some feel that the healing time for CNS injuries may take longer than non-CNS injuries, such as in the skin and pulmonary tissues [
10]. Importantly, it has been determined that there was not replication-competent SARS-CoV-2 isolated after three weeks in acute COVID-19 infections [
12,
13].
Peripheral inflammation of the immune–brain axis is thought to be bidirectional in humans in regards to immune system function. COVID-19 has been shown to have a dysregulated immune system response to SARS-CoV-2 and has highlighted the important role of cytokines and chemokines since it is associated with a profound hypercytokinemia that has been termed cytokine storm [
14]. Cytokines may be defined as a broad and loose category of small immunomodulatory peptides, proteins, or glycoproteins (~5–30 kDa) which are important in cell signaling including growth, maturation, and responsiveness of particular cell populations, and chemokines are cytokines with chemotactic properties that induce leukocyte migration and transmigration across brain endothelial cells (BECs) in the CNS [
15].
This review will focus on the peripheral pro-inflammatory cytokines/chemokines (PCC) and the central nervous system (CNS) proinflammatory cytokines/chemokines (CNSCC) and their effects on the neurovascular unit (NVU) blood–brain barrier (BBB) and the choroid plexus (CP) blood–cerebrospinal barrier (BCSFB), neuroglial cells (microglia cell(s) (MGCs), astrocytes (ACs), and oligodendrocytes (OLGs)); the response to injury wound healing mechanisms; and LC/PASC chronic neuroinflammation. In addition to the acute COVID-19 inflammatory thromboembolic state, the effect of chronic inflammation in CNS and its associated microthrombi/microbleeds, which result in decreased cerebral blood flow (CBF) and ischemia and ischemia/reperfusion injury to evoke even further neuroinflammation to result in LC/PASC, will be discussed. Furthermore, the importance of impaired folate-mediated one-carbon metabolism will be discussed and related to the LC/PASC post-COVID-19 syndrome in addition to the possibility of hypoadrenalism.
There is a paucity of COVID-19 autopsied brain tissue that have been properly prepared to study ultrastructure changes. However, the changes seen among neuroinflammatory conditions likely have a number of similarities. One of the best studied conditions from the ultrastructural point of view that has a neuroinflammatory component are the models of type 2 diabetes (T2DM). Therefore, transmission electron microscopy (TEM) works on models of T2DM (db/db and BTBR ob/ob) which are utilized to illustrate stress-induced ultrastructural remodeling. These TEM images are utilized in order to be merely representitive of the possible similar stress injury-induced remodeling changes that may also be present in LC/PASC as a result of previous COVID-19 infections with neuroglial microglia, astrocyte activation, and neuroinflammation.
2. Peripheral—Pro-Inflammatory Cytokines/Chemokines (PCC), Brain Barriers and CNS Cytokines/Chemokines (CNSCC): Mechanisms of Chronic Neuroinflammation and Impaired Cognition in LC/PASC
Because SARS-CoV-2 binds to the cellular angiotensin converting enzyme 2 (ACE2) receptor that is present in multiple organs systems, LC/PASC has a multifactorial pathogenesis which incorporates chronic inflammation both in the peripheral organ systems as well as in the CNS [
16]. Neuronal system dysfunction, specifically including the activation of neuroglial cells (microglia and astrocytes), is associated with neuroinflammation and impaired cognition, and the activation/dysfunction of brain endothelial cells are associated with subsequent inflammatory thromboembolism. Thus, the activation of neuroglial microglia and astrocytes are thought to be key pathogenetic mechanisms in LC/PASC [
16].
The
PCC’s are thought to be elevated in LC/PASC individuals and they may occur in a continuous process or in recurrent wave-like processes that affect the brain (
Figure 3) [
16,
17].
Phetsouphanh et al. [
17] have presented findings from the adaptation of evidence-informed complex population health interventions for implementation and/or re-evaluation in new contexts (ADAPT) study that demonstrated a uniquely prolonged effect on aspects of both the innate and adaptive immune systems. They further suggested that these findings may be driving the LC/PASC syndrome. They found elevated type I interferons and type III interferon levels at eight months in LC/PASC and suggested that these findings demonstrated ongoing inflammation in contrast to those without LC/PASC [
17]. Additionally, they studied a prospective cohort of individuals with LC/PASC (the ADAPT study) and compared results to age/gender-matched subjects without LC/PASC, healthy donors, and individuals infected with other non-SARS CoV2 human coronaviruses (the ADAPT-C study) and found an elevated diffuse serum inflammatory cytokine profile in symptomatic long COVID subjects that was maintained at eight months post-infection, which was not observed in asymptomatic COVID-19 survivors. This elevated cytokine profile in LC/PASC individuals consisted of an elevation of type I and III interferon levels, which are considered to be antiviral cytokines [
17]. Interestingly, type I interferons (IFN) consist of IFN-α, IFN-β, IFN-ε, IFN-κ, and IFN-ω that, in humans, bind to the ubiquitously expressed type I IFN receptor (IFNAR), and type III interferons consist of IFN-λ1 (IL-29), IFN-λ2 (IL-28a), and IFN-λ3 that bind to the type III receptor (IFNLR) that are preferentially expressed on epithelial and certain myeloid cells [
18].
Additionally, Low et al. have examined the role of
PCC and binding to the brain endothelial cell receptors with subsequent activation of the blood–brain barrier, brain endothelial cells, pericytes, and astrocytes in producing and contributing to increased
CNSCC, which result in chronic neuroinflammation as well as the additional generation of reactive oxygen/nitrogen species (RONS) [
19]. Importantly, oxidative stress and RONS induce neuroinflammation via activation of nuclear factor kappa B (NF-κB) with the creation of a RONS–vicious inflammatory cycle since neuroinflammation begets RONS in the central nervous system and RONS begets neuroinflammation [
20]. Furthermore, during the earlier acute COVID-19 phase the central nervous system microglia cells may be already primed and capable of elaborating even more cytokines and fueling this vicious cycle between RONS and neuroinflammation with increased
CNSCC. These mechanisms may result in even more chronic ongoing neuroinflammation and result in central nervous system-mediated LC/PASC with central nervous system symptoms of fatigue, malaise, fever, impaired cognition, memory loss, dysphoria, and depression associated with LC/PASC [
21]. While papers by Phetsouphanh et al. and Low et al. are currently in preprint format, they are each strong papers and are highly referenced [
17,
19].
2.1. Peripheral Proinflammatory Cytokine/Chemokine (PCC) Effects on the Neurovascular Unit (NVU): Activation of Neuroglia (Microglial, Astrocytes and Oligodendrocytes)
As the peripheral systemic
PCC perfuse the cerebral microcirculation, they will attach to their respective specific receptors on brain endothelial cell(s) (BECs) and, in turn, will activate BECs, pericytes, and astrocytes that comprise the NVU blood–brain barrier interface. In turn, these NVU cells will release central nervous system-derived cytokines/chemokines (
CNSCC) resulting in the activation of the neuroglia, consisting of the central nervous system microglial cells, astrocytes, and oligodendrocytes, which will also add to the overall burden of increased
CNSCC and neuroinflammation [
21]. Further, Erickson et al. [
22] have demonstrated the transport of the chemokine CCL11 across the BBB, and Quaranta et al. have recently presented a poster entitled: Interactions of Chemokines with the Blood–Brain Barrier at the American Society for Pharmacology and Experimental Therapeutics (ASPET) conference, which demonstrated that the CCL2 and CCL5 can cross the BBB by a non-saturable transport system (personal communication with Daniel V. Quaranta of the Banks and Erickson laboratory).
PCC are known to modulate the inflammatory immune response throughout the body and a dysregulation or excessive production that occurs in the acute COVID-19 cytokine storm along with the residual
PCC are known to be a central feature for the development of central nervous system neuroinflammation, neurodegeneration, and dysmyelination including myelin remodeling in the central nervous system via activation of the neuroglia and their addition to the
CNSCC pool (
Figure 3) [
23].
2.2. Microglia Cells (MGCs)
Microglia cells (MGCs) are the mesodermal yolk-sac-derived resident innate immune cells of the brain and possess a unique quality to undergo rapid changes in their diversified spectrum of varying ultrastructural phenotypes. They may exist in a ramified state, a polarized activated amoeboid state, and an aged senescent morphological state [
24]. Ramified microglia cell(s) (rMGCs) (M-2 polarization-like state) serve continuously as the housekeepers and cleaners of normally accumulating cellular and metabolic debris. They are the innate immune cells of the central nervous system and are in constant surveillance of their surroundings in order to detect any type of damage or injury within the central nervous system including the neurovascular unit and the blood–brain barrier (
Figure 4 and
Figure 5A) [
24].
Ramified microglia cells (rMGCs) stand ‘on guard’ and are ready at a moment’s notice to morphologically shape shift into a M-1 polarization amoeboid-activated microglia (aMGCs) (
Figure 5B,C), wherein their elongated cytoplasmic extensions become shorter, thicker, and more mobile. Additionally, there are changes within the aMGCs mitochondria in that they become increased in number, swollen, lose their mitochondria electron density within the matrix and fragment smudge, or lose their cristae. Normally microglia cells are in a ramified cleaning state and are in constant surveillance of their CNS surroundings including the neurovascular unit blood–brain barrier interface (
Figure 4); however, when they are activated via
PCCs/
CNSCCs they will become activated to an M-1-like phenotype—aberrant MGCs (aMGCs) (
Figure 5B,C) and become capable of generating reactive oxygen/nitrogen species, proteases, and will also add to the pool of increased secreted
CNSCC [
24]. Importantly, the aMGCs are responsible for the rearrangement attenuation and/or loss of the tight and adherens junctions (TJ/AJ) of the neurovascular unit blood–brain barriers of brain endothelial cells. This perturbation of the brain endothelial cells allows for increased adherence and transcellular and paracellular transmigration of innate immune neutrophils and monocytes (monocyte-derived macrophages with the brain) and adaptive immune lymphocytic cells into the central nervous system [
24,
25]. Interestingly, rMGCs can be primed by any central nervous system inflammatory insult and, upon being rechallenged, these primed MGCs are capable of becoming hyperactive aMGCs (capable of undergoing even further spectral changes) that produce even more toxic
CNSCC and reactive oxygen nitrogen species than when they were initially activated the first time and, thus, implies a two-hit hypothesis [
24,
26]. This two-hit hypothesis may be helpful to explain why some COVID-19 patients, such as those with obesity and T2DM, are at greater risks for the development of more severe complications related to COVID-19, i.e., their microglia cells may be already primed from exposure to a chronic low-grade inflammation arising from adipose tissue depots, and COVID-19 will act as a second hit via an increase in
PCC. The microglial cell two-hit hypothesis responsible for the hyperactive/exaggerated response of previously sensitized or primed aMGCs may also be in play in those with LC/PASC and help to explain some of their delayed neurological symptoms [
26].
2.3. Astrocytes (ACs)
Astrocytes (ACs) are the most abundant cell in the human brain and with their vast connections to multiple other cell types including themselves are the master connecting and communicating cells within the human brain (
Figure 6) [
25,
27,
28,
29]. This large AC cellular presence in the brain with their vast cell–cell communications via gap junctions may be viewed as the brain’s functional syncytium [
25,
30].
Astrocytes perform multiple important functions in the brain to provide maintenance of extracellular homeostasis, including glutamate uptake and recycling; potassium buffering; supply of energy substrates, especially the astrocyte neuron coupling involving the astrocyte neuron lactate shuttle (ANLS) hypothesis; spatiotemporal neurovascular unit coupling between neuronal activity; and cerebral blood flow, providing functional hyperemia (
Figure 5B,C and
Figure 6), pH buffering, and antioxidant defense against oxidative stress via glutathione (GSH); and recycling of the micronutrient ascorbate, to name a few [
31].
Peripheral immune activation, such as
PCC, can instigate rapid and concerted responses of
CNSCC neuroglia regulated through fine-tuned communications between astrocytes, microglia, and mural cells of brain barriers including brain endothelial cells and pericytes. This also propagates inflammatory signals over the brain, and induces physiological and, importantly, behavioral changes that may correspond to sickness behavior or more severe impaired cognition characteristic of the LC/PASC, as noted in more severe sepsis associated encephalopathy models [
32]. One remodeling characteristic of astrocyte activation in the diabetic db/db model was detachment and retraction from the basement membrane of the neurovascular unit, which could result in the uncoupling of the neurovascular unit and impair regional vasodilation when regional neurons are active and, thus, impair regional cerebral blood-flow-producing regional hypoxia (
Figure 5B,C) [
25]. Reactive astrocytes following injury are initially protective; however, as the response to injury progresses, the astrocytes will remodel to become a more astrogliopathic phenotype with retraction of their cytoplasmic extensions and lose their communication with not only the neurovascular units but also neurons, synapses, and their own cell–cell connections [
25,
32].
2.4. Oligodendrocytes (OLGs) and Myelin
Oligodendrocytes (OLGs) play an important role in myelin synthesis, wrapping, and compaction of myelinated axons and are remodeled when there is injury by T2DM with excess of reactive oxygen/nitrogen species and
CNSCC [
33]. Further, activation of microglia and astrocytes will also have effects on the function of the OLGs. Previously, our lab has found prominent remodeling of OLGs with increased nuclear chromatin condensation and volume with increased numbers of active myelination sites of the cytoplasm in transition zones. Furthermore, marked aberrant myelination was noted with outer myelin lamellae sheath with splitting, separation, ballooning, and aberrant mitochondria in grey matter cortical layer III and similar myelin remodeling changes with marked disarray with additional axonal collapse in transitional zones between the grey and white matter in the diabetic
db/db as compared to the non-diabetic controls at 20-weeks of age (
Figure 7) [
33].
Oligodendrocytes, oligodendrocyte precursor cells, and myelination are not static and readily adapt to changes in their environment. The previously described alterations in myelin remodeling in the diabetic
db/db models may be detrimental and could impair cognition by the slowing of transmission rates of information and action potentials in white matter tracts to distant regions. This aberrant remodeling could result in a loss of synchronicity, not only between neurons, but to entire neuronal networks [
33]. Importantly, activated astrocyte foot process (end-feet) separation and retraction from neuronal synapses and myelinated neurons, as in diabetic db/db models (
Figure 6 Insert 2 and
Figure 7F), could interfere with the supply of energy substrates (glucose and lactate) to neurons, neuronal synapses and neuronal axons (myelinated or unmyelinated). This is especially true in regards to the astrocyte neuron lactate shuttle (ANLS) hypothesis as the astrocyte neuron coupling of the ANLS hypothesis would be interrupted due to separation and retraction [
31,
32,
33,
34].
3. PCC Effects on the Vulnerable Choroid Plexus (CP) Blood-Cerebrospinal Fluid Barrier (BCSFB)
The choroid plexus (CP) resides within each of the four ventricles and secretes and purifies the cerebrospinal fluid (CSF) that acts as a buffer to reduce traumatic brain injuries, provides nourishment, and removes neurotoxic waste. It is also important in controlling intracranial pressure, maintaining CSF ion homeostasis, and providing micronutrients vitamins, proteins and hormones for neuronal and glial development, maintenance, and function. Further, it is important in secreting numerous trophic/stabilizing proteins (brain-derived neurotrophic factor), transthyretin, and newly described choroidal phenomena, which includes the integration of circadian clock signals, immune interaction with gut microbiome, and expression of receptors to taste are now being explored even on a molecular level [
35,
36].
The
PCCs will have a similar effect on the choroid plexus blood–cerebrospinal fluid barrier (BCSFB) as in the neurovascular unit blood–brain barrier in that they will activate the stromal fenestrated endothelial cells and the stromal cell population including macrophages, fibrocytes, and dendritic cells that reside in the space between the fenestrated capillaries and the basilar ependymal epithelial cell(s) (EPYs). EPYs have tight and adherens junctions (TJ/AJ) between adjacent ependymal cells of the choroid plexus located at the apical cell–cell interface on the cerebrospinal fluid side and are responsible for the choroid plexus barrier functions. (
Figure 8) [
35,
36,
37].
The choroid plexus is important for many reasons including its ability to synthesize and secrete and purify the CSF, important for maintaining the intracranial pressure and maintaining ion and acidity (pH due to bicarbonate transporters). Importantly, the choroid plexus provides for micronutrient and vitamin supply, which may play a crucial role in individuals with LC/PASC since some of these patients may have a marked decreased in micronutrients, proteins, and hormones, such as leptin, thyroxin, and progesterone for neuronal and glial development, maintenance, and function [
35,
36,
37] as a result of their acute episode with COVID-19 and lack of oral micronutrients, vitamins, and protein while in an intensive care environment or due to a lack of appetite and proper oral nutrient intake.
Importantly, the choroid plexus is more vulnerable to become injured by neurotoxins as compared to the neurovascular unit blood–brain barrier since its stromal capillaries are fenestrated and its ependymal tight junctions/adherens junctions are not as tight as compared to the brain endothelial cells of the blood–brain barrier (
Figure 9) [
35,
36,
37,
38,
39,
40,
41].
The choroid plexus contains fenestrated capillaries surrounded by a stromal space where immunocompetent macrophages, fibroblasts, and dendritic cells are located, and each of these stromal cells are capable of responding to an elevated level of
PCCs and contribute to even more central nervous system-derived cytokines and chemokine (
CNSCC) within the brain, since the choroid plexus blood–cerebrospinal fluid barrier, similar to the blood–brain barrier, are each neuroimmune axes [
21]. Importantly, it has been recently found that the choroid plexus undergoes extensive remodeling when exposed to stress/injury such as T2DM in the diabetic BTBR ob/ob model (
Figure 10) [
37].
The choroid plexus has proven to be a region within the brain that is known to stain positive for ACE2 (the receptor for SARS-CoV-2) and is also known to stain positive for remanent proteins of the SARS-CoV-2 both in organoids and in autopsy case reports [
42]. The choroid plexus has been long overlooked as a major barrier in the central nervous system, while the blood–brain barrier remains the primary concern for most researchers.
There are those who currently think that the choroid plexus is equally important as the blood–cerebrospinal fluid barrier since these two barriers seem to coordinate their functions, while the choroid plexus blood–cerebrospinal fluid barrier acts both as a gateway and a target [
43] in allowing toxic substances, such as excessive toxic
PCC and immune cells, to enter the cerebrospinal fluid and eventually into the central nervous system neuropil extracellular space containing the interstitial fluid. Interestingly, the purifying action of the cerebrospinal fluid is largely due to the finding that it turns over almost three times a day due to the highly vascular choroid plexus microvilli [
35,
36].
Importantly, neuroepithelial cells that line the neural tube during embryological development eventually become the ependymal lining cells of the cerebrospinal fluid ventricles. These neuroepithelial cells develop both tight and adherence junctions between adjacent ependymal cells to provide the barrier function at the paracellular location and, thus, the choroid plexus barrier function of ependymal cells are well-developed at the time of birth. Interestingly, the neuroepithelium is the original epithelial cell lining the neural tube during embryologic development. This suggests the possibility of these lining ependymal epithelial cells that originated from the neuroectoderm may become a possible regional niche or repository for a future source of stem cells later in life. It is known, for example, that the subventricular zone (SVZ) provides a rich source of progenitor cells including neurons and glial cells in adult humans. For a more in-depth examination of the embryological development of the choroid plexus, one should consider reading the paper by Liddlow [
43].
During the preparation for this manuscript, an important paper was published by Yang et al [
44]. This group led by Wyss-Coray demonstrated that the vulnerable choroid plexus may harbor a definite entry point of peripheral inflammation, including
PCC, that are capable of signaling both neuroglial cells (microglia and astrocytes) to contribute to neuronal cell and synaptic dysfunction. Herein, their observations from their abstract are quoted below:
“We observe broad cellular perturbations which predict that choroid plexus barrier cells sense and relay peripheral infammation into the brain and show that peripheral T cells infiltrate the parenchyma. We discover COVID-19 disease-associated microglia and astrocyte subpopulations that share features with pathological cell states reported in human neurodegenerative disease 4–6. Synaptic signaling of upper-layer excitatory neurons—evolutionarily expanded in humans 7 and linked to cognitive function 8 are preferentially affected in COVID-19. Across cell types, COVID-19 perturbations overlap with those in chronic brain disorders and reside in genetic variants associated with cognition, schizophrenia, and depression. Our findings and public dataset provide a molecular framework to understand COVID-19 related neurological disease observed now and which may emerge later.”.
The above references used in this quotation will also be placed in this review [
45,
46,
47,
48,
49].
4. The Importance of an Endothelial Glycocalyx (ecCGx) and Epithelial Glycocalyx in Brain Barriers in Relation to LC/PASC
An intact glycocalyx of the endothelial and epithelial cells is of great importance in brain barrier homeostasis (
Figure 11).
The endothelial glycocalyx (ecGCx) is vasculoprotective and acts as the first barrier of the tripartite BBB and in the stromal fenestrated endothelial capillaries of the blood–cerebrospinal fluid barrier (BCSFB). Furthermore, the epithelial ependymal cells of the choroid plexus are covered by a surface layer, the epithelial glycocalyx. Additionally, the ecGCx serves as a protective coating and, thus, prevents the direct contact of the circulating blood and its constituents with the plasma membrane of the brain endothelial cells. This gel-like mesh network is responsible for multiple other protective functions in the vasculature including the promotion of vascular integrity and homeostasis, signaling blood and vessel wall capillary interactions, providing an anti-inflammatory, antithrombotic, and anti-contractile surface layer, limiting access of certain molecules based on size, steric hindrance, and especially electrostatic charge, since the glycocalyx has a strong negative net charge and is capable of repelling highly negative charged molecules and mechanotransduction of fluid shear stress and subsequent maintenance of bioavailable nitric oxide to the vessel wall. Importantly, the ecGCx is capable of modulating inflammatory processes via its prevention of leucocyte and platelet adhesion [
9,
24,
28,
37,
50,
51]. Furthermore, an epithelial glycocalyx is known to be present and adheres to the microvilli of the ependymal epithelial cells that line the ventricles and specifically the choroid plexus ependymal cells at the apical CSF interface (
Figure 8C,D,
Figure 10B and
Figure 11) [
37]. Further, the shedding of the glycocalyx in response to inflammatory mediators, such as cytokines and chemoattractants, was found to occur in arterioles, capillaries, and venules under various experimental models of inflammation [
51].
It is also important to note that the ecGCx and epithelial glycocalyx are specialized extracellular matrices containing numerous proteoglycans and that synapses in the central nervous system are surrounded by a complex assortment of protective proteoglycans, such as chondroitin sulfate proteoglycans (CSPGs), tenascin-R (TN-R), hyaluronan (HA), and link proteins termed the perineuronal net(s) (PNNs) [
52]. These PNNs are comprised of numerous compressed scaffolding of synapses and are certainly very vulnerable to the detrimental effects from proinflammatory
CNSCC that are associated with increased oxidative stress (RONS) provided by the proinflammatory (activated microglia and the reactive activated astrocytes). PNNs have multiple roles but the most critical are the regulation of synaptic plasticity via stabilizing synapses and, further, provide protection of neurons and synaptic connections against damaging stressors such as the increased damaging proinflammatory
CNSCCs and reactive oxygen nitrogen species that result from activation of the activated neuroglia effector cells (activated microglia and activated astrocytes) [
51,
52,
53]. Notably, PNNs are also involved in cognition, which include encoding, maintaining, and updating memories as well as neuroglia activation by
PCC and
CNSCC may be involved to promote the impaired memory, focus, “brain fog”, and impaired cognition in LC/PASC [
53,
54].
5. Compromised Folate-Mediated One-Carbon Metabolism (FOCM) in LC/PASC: Importance of Hyperhomocysteinemia (HHCY) and Deficient Micronutrients (Vitamins B12 and B9)
Homocysteine (Hcy) is a nonessential sulfur-containing amino acid and an intermediary metabolic product derived from the demethylated essential amino acid methionine [
20]. It is commonly known that plasma concentrations of homocysteine are inversely related to plasma concentrations of folate, vitamin B12, and vitamin B6, as well as to the intake of these vitamins [
20,
55,
56,
57,
58,
59]. Further, it is now accepted that hyperhomocyteinemia (HHCY) has vasculotoxic effects as well as neurotoxic effects that are associated with neuroinflammation, neurodegeneration, pro-oxidation, as well as proatherogenic/prothrombotic effects [
20,
55,
56,
57,
58,
59,
60]. Elevations of Hcy or hyperhomocysteinemia (HHCY) plays an important role in the causation of oxidative stress with excess formation of reactive oxygen nitrogen species in the endothelium and in the central nervous system due to autoxidation of Hcy, formation of Hcy mixed disulfides, and interactions of Hcy thiolactones and protein homocysteinylation [
20,
60,
61,
62,
63].
Accumulating knowledge has recently demonstrated that HHCY is an independent risk factor for cognitive dysfunction [
59,
61,
62,
63,
64]. Both indirect and direct vascular damage can be caused by elevations in homocysteine, and HHCY has been implicated in vascular dementia with an increased risk of multiple brain infarcts and dementia as homocysteine levels rise [
65,
66]. Indeed, hypomethylation of DNA, proteins, and fats may render the cerebral vascular structures as well as neurons that are more vulnerable to inflammatory damage (such as
PCC and
CNSCC) and even apoptosis. These observations of HHCY and decreased vitamin B12, B9—folic acid, and other B vitamins emphasize the proper functioning of folate-mediated one-carbon metabolism (FOCM) typified by the methionine–folate cycle and the transsulfuration pathway. Their essential effects on cognitive diseases are of great importance as they not only apply to acute COVID-19 but, even more importantly, to LC/PASC syndrome (
Figure 12) [
58,
59,
60,
61,
62,
64,
65,
66,
67].
Micronutrients consist primarily of vitamins and minerals and play an important role in CNS homeostasis. Micronutrients are an essential component of several general cellular functions in addition to proper functioning of neurologic activity, such as the synthesis of certain neurotransmitters including dopamine, serotonin, myelin formation, synaptic plasticity, and energy production, which is decreased in many neurodegenerative diseases [
65,
67].
When there is insufficient uptake or depletion of micronutrients, the brain cannot continue to function normally and the result will be dysfunction or disease, i.e, a loss of homeostasis, such as in the dysfunction of FOCM which occurs in both acute COVID-19 and post-COVID-19 LC/PASC [
68]. Furthermore, as SARS-CoV-2 infects host cells, it high-jacks the normal one-carbon cellular metabolic machinery. This places significant demands on the host cell methyl-groups (-CH3) in order to replicate its ribonucleic acid (RNA) and provide RNA capping to produce large numbers of progeny virions. Therefore, SARS-CoV-2 infection leads to increased demands of the host cells one-carbon methyl-groups via its excess in
PCC and
CNSCC and the associated increase in redox-oxidative stress (reactive oxygen nitrogen species) to promote other disturbances of one-carbon metabolism. Additionally, SARS-CoV-2 may disrupt the coordination between remethylation and transsulfuration through inadequate amounts of the methyl-donor SAM. Furthermore, there may be serine depletion in addition to elevated Hcy and glutathione (GSH) depletion and the dysfunctional oxidized cofactor cobalamin (vitamin B12). Briefly, it appears that SARS-CoV-2 not only stresses the host cell one-carbon metabolism but also impairs the supply of methyl groups provided by SAM (
Figure 12) [
68].
Zhang et al. have been able to elegantly demonstrate that, in addition to the SARS-CoV-2 hijacking of one-carbon metabolism, it also hijacks folate (folic acid), and that folate metabolism is absolutely critical for the transfer of one-caron units for nucleotide synthesis in addition to its important role in glutathione synthesis via the transsulfuration pathway [
69]. In addition, Schober et al. have demonstrated that the one-carbon pool controls mitochondrial energy metabolism via complex I and iron-sulfur clusters [
70].
COVID-19 is now widely known to be associated with excess PCC and CNSCC, and this cytokine storm is associated with oxidative redox stress (reactive oxygen nitrogen species). This combination of cytokine storm and oxidative redox stress has a marked detrimental effect on the methionine cycle and its methionine synthase (MS) that converts Hcy to methionine and especially its essential cofactor vitamin B12-cobalamin. When essential cobalamin is oxidized from its functional cob(I)alamin species to the oxidized cob(II)alamin species, it will not run the MS reaction to produce methionine from Hcy and, in turn, will not be able to produce the major methyl-donor group S-adenosyl methionine (SAM) that is necessary for the host cells requirements for one-carbon metabolism.
As one begins to better understand the impaired one-carbon metabolism hypothesis in COVID-19, it becomes apparent that supplemention to patients suffering from LC/PASC with vitamin B12 and folate might have positive responses due to the impairments caused by this syndrome. For example, Regland et al. demonstrated that individuals suffering from myalgic encephalomyelitis and fibromyalgia, i.e., chronic fatigue syndrome with symptoms similar to LC/PASC, had a positive response of their symptoms when treated with injectable B12 and oral folic acid [
71]. Further, accumulation of aberrant dysmorphic and dysfunctional aberrant mitochondria (aMt) in key cells including endothelial cells, pericytes, astrocyte foot processes of the neurovascular unit, protoplasmic and fibrous astrocytes, oligodendrocytes, and myelinated or unmyelinated axons, as noted in diabetic db/db models, may be related to impaired one-carbon metabolism.
7. COVID-19 Effects on the Hypothalamic-Pituitary-Adrenal Axis (HPA) and Adrenal Gland with Residual Effects in LC/PASC
In response to critical illnesses and marked psychogenic stress (including physical, emotional, or immunological stresses, such as excess
PCC), the hypothalamic–pituitary–adrenal (HPA) axis is able to orchestrate the systemic release of cortisol in human and corticosterone in rodents referred to as glucocorticoids (generally considered anti-inflammatory immunomodulators) [
74]. The brain is known to respond to peripheral systemic cues, such as inflammation and the increased
PCC that occurs due to the cytokine storm in acute COVID-19 and possibly LC/PASC. While cytokines and chemokines are known to have receptors on brain endothelial cells in the neurovascular unit blood–brain barrier and the blood–cerebrospinal fluid barrier, they are also known to signal the hypothalamus early on in COVID-19 via the leaky fenestrated circumventricular organ (CVO) capillaries (
Figure 14).
Further, stress-induced glucocorticoids are an essential response to survive severe intensive care unit (ICU) illnesses, such as COVID-19 with SARS-CoV-2, as well as the excessive emotional and burdening stresses placed on the patients in these extreme stressful situations. Indeed, this stress-induced glucocorticoid secretion in severe disease may result in a relative adrenal insufficiency state [
75].
In a series of autopsy studies, Zinserling et al. have been able to demonstrate in COVID-19 decedents that the adrenal gland may be infiltrated with CD3+ and CD8+ lymphocytes in perivascular regions due to SARS-CoV-2, resulting in impaired adrenal function [
76]. The lymphocytic vasculitis they described would also increase the risk of the known endotheliitis that is associated with SARS-CoV-2 and, in turn, increase the inflammatory thromboembolic state within the adrenal that has also been reported by Santana et al in their autopsy studies [
77]. Santana et al. reported frequent adrenal lesions consisting of an increase in ischemic necrosis, thrombus formation, hemorrhage, and cortical lipid degeneration with these abnormalities in 12 of 28 patients autopsied [
77].
Hypoadrenalism and adrenal insufficiency may be plausible in LC/PASC [
75]. Siejka and Barabutis [
75] have proposed at least three mechanisms for the possible development of adrenal insufficiency due to SARS-CoV-2 as follows: (i) relative adrenal insufficiency is thought to be a common condition in critically ill patients [
78] and increased
PCC/
CNSCC in acute COVID-19 may over time result in a negative feedback toward the hypothalamic-pituitary-adrenal axis [
79]; (ii) SARS-CoV-2 generates certain amino acid sequences that may mimic the hosts adrenocorticotropic hormone (ACTH) and, as a result of these corresponding antibodies, may contribute to the development of central adrenal insufficiency [
75]; and (iii) the pituitary, hypothalamus, and adrenal glands each express angiotensin-converting enzyme 2 (ACE2), and SARS RNA has been identified in hypothalamic tissues of autopsied patients with COVID-19 that might cause hypophysitis and adrenalitis, and promote either secondary or primary adrenal insufficiency [
75,
80]. Therefore, LC/PASC patients should be carefully evaluated for adrenal insufficiency.
8. Central Nervous System Inflammatory Induced Thromboembolism: Microclots and Microbleeds in Acute COVID-19 and Possibly LC/PASC Due to Increased PCC and CNSCC
Systemic
PCC and CNSCC in COVID-19 are known to induce endothelial cell activation and an inflammatory related immunothrombotic or thromboembolic state within the vasculature. This immunothrombotic state ultimately causes macro-microthrombotic complications in multiple organ systems including the central nervous system, which may relate to either macrovascular (arteries and veins ≥ 200–300 μm) or microvascular (arterioles, venules or capillaries ≤ 20 μm) thrombi or clots [
81].
Microvascular thrombi may also be associated with microbleeds especially in the white matter regions. These thrombi or microbleeds associated with cerebral small vessel disease (cSVD) will result in ischemic injury and ischemia/ischemia reperfusion injury due to decreased cerebral blood flow and impaired metabolic delivery and metabolic byproduct uptake that may induce even further neuroinflammation due to increased CNSCC. This increase in CNSCC will develop in a response to injury wound healing mechanism due to activated neuroglia (activated microglia and reactive activated astrocytes) which secrete CNSCC and reactive oxygen nitrogen species, resulting in ischemia-related regional injuries due to decreased cerebral blood flow to evoke even further neurodegeneration and or impaired cognition (
Figure 5 and
Figure 15) [
82].
Importantly, endothelial cell activation and dysfunction are tightly associated with cSVD and the endothelial activation in acute COVID-19 and LG/PASC with endotheliitis may certainly be playing a role with the development of microbleeds and/or clots [
9,
82].
Additionally, platelet activation due to endotheliitis with endothelial cell activation and dysfunction may also be a contributing factor since Pertorius et al [
83] have demonstrated that, in LC/PASC, there is a persistent clotting protein pathology with increased thrombosis and an impaired fibrinolytic system resulting in impaired fibrinolysis that is associated with amyloid formation and increased antiplasmin [
83,
84]. Interestingly, serum amyloid A (SAA) is an amyloidogenic/fibrillogenic acute phase protein synthesized by the liver in inflammatory states (acute and chronic) that can affect coagulation by inducing amyloid formation in fibrin(ogen), as well as by inducing platelets to a more prothrombotic state [
83]. Further, endothelial and platelet activation with a known endotheliitis [
85] due to elevated
PCC in acute COVD-19 may take a while to resolve, especially if there is an ongoing elevation of
PCCs.
Recently, Chioh et al. were able to demonstrate that convalescent COVID-19 patients were susceptible to endothelial cell dysfunction due to persistent immune activation [
86]. They were able to identify circulating endothelial cells in convalescent patients who had had COVID-19. Additionally, this group suggested that endothelial instability, damage, and activation/dysfunction may play a key mechanism that could be responsible for the development of post-COVID-19 vascular prothrombotic complications. Their findings, in combination with the previous discussion regarding increased inflammatory-induced thromboembolism, could also implicate an increased risk for the development to microclots and/or microbleeds, as discussed previously [
81,
85,
86]. Further, they suggested that there may be a vicious cycle in regards to endothelial inflammation via
PCC, vascular leakage, activation of the coagulation pathway, and inflammation. Certainly, the findings by Chioh et al. need to be carried out to longer time frames post-COVID-19 presentation.
10. Conclusions
It has been recently estimated (May 2021) that between 10% to 35% of patients not requiring hospitalization for acute COVID-19 may develop post-COVID-19 symptoms, regardless of co-morbidities [
1,
16,
88], while incidence rates of up to 70% have been reported among hospitalized patients and among patients with severe illnesses [
89]. The true prevalence of LC/PASC syndrome is currently unknown; however, it has been stated that as many as one-in-three survivors or more depending on the depth and reliability of investigation may develop LC/PASC [
89].
Some are now concerned, and rightfully so, that a significant numbers of patients who have had COVID-19 may develop myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), wherein they calculated that roughly only 10% would develop ME/CFS of approximately 25 million by the end of 2021, leaving an approximated total of 2.5 million who would meet the standards for the National Academies of Sciences, Engineering, and Medicine (NASEM) case definition criteria for ME/CFS in the United States [
90]. If their projections are anywhere close to their approximated figures this occurrence, similar to the historical Spanish flu pandemic, this would indeed be another historic event in the history of chronic infection-related illness.
While the COVID-19 pandemic has caused great morbidity and mortality across the world, many have survived its acute effects and a large number of those survivors, even those with only minor acute symptoms, are now experiencing a debilitating and prolonged post-viral syndrome termed LC/PASC. This review has not only discussed and demonstrated the important role of the neuroglia cells as first responders, protectors, and gate-keepers of the CNS, but also emphasized their important role as the final effector cells in the response to injury wound healing mechanism due to the increased PCCs. Each of the neuroglial cells remodeling changes have been described in response to central nervous system (CNS) injury. Further, these neuroglial ultrastructural remodeling changes result in CNS abnormalities that are compatible with the multiple complicated neurological and neuropsychiatic symptoms associated with acute COVID-19 as well as the post-COVID-19 viral syndrome of LC/PASC. Currently, we are only at the cusp of understanding the multiple pathobiological mechanisms involved in the development of the multifactorial syndrome of LC/PASC.
The importance of increased
PCCs with acute COVID that activate
CNSCCs would act as an injury and a response to injury wound healing mechanism would activate neuroglia (microglial cells and astrocytes) to negatively interact with neurovascular structures, CNS neurons, and contribute to neuroinflammation and its contribution to impaired cognition was noted. The neuroglia via the activation of microglial cells and astrocytes carries out their role as a result of being the primary effector cells responsible for the CNS remodeling changes in the brain that may be associated with so many of the neurological symptoms in LC/PASC. Certainly, viremic hematogenous and neural routes for the direct viral entry into the brain still remain a strong possibility; however, at this point in time, there are only a few case reports of actually finding the virus in the central nervous system tissues or even in the cerebrospinal fluid. This may change as we continue to learn more about the direct impact of SARS-CoV-2 on the brain from various animal models and larger studies forthcoming from autopsied brain studies. One such study recently revealed that the S1 protein of SARS-CoV-2 is capable of entry into the central nervous system via the brain endothelial cells of the intact neurovascular unit blood–brain barrier by adsorptive transcytosis and that the murine angiotensin-converting enzyme2 (ACE2) is involved in brain uptake [
91]. Therefore, we need to keep an open mind to future findings and incorporate them to what is currently known.
Still, it seemed overwhelming that the PCCs would be capable of causing these neurologic symptoms via activating the effector neuroglial cells and instigating an ongoing neuroinflammation. Importantly, we know that there are cytokine and chemokine receptors on the BECs of the neurovascular unit blood–brain barrier, the brain endothelial cells of the choroid plexus blood–cerebrospinal fluid barrier villous protrusions. and the ultimate epithelial ependymal barrier of the choroid plexus blood–cerebrospinal fluid barrier. This review has attempted to show that neuroglia activation by PCC and CNSCC and chronic neuroinflammation may be the common denominator for the development of the complex LC/PASC syndrome following the acute COVID-19 due to SARS-CoV-2.
Understanding the ultrastructure of the brain is important to provide a better understanding of the central nervous system’s normal and abnormal ultrastructure and how remodeling changes are associated with function, such as when studying the effects of a stressor or injury, whether it is due to central nervous systems neuronal diabetic stress/injury or understanding the stress/injury that may also be associated with the ultrastructural remodeling changes due to the stress/injury in acute COVID-19 and/or the remodeling stress/injury of LC/PASC. TEM images allow one to discern details of the brain parenchyma at micrometer and nanometer resolution and their unique features in two-dimensional images. These TEM images allow us to be able to distinguish neurons and their supportive neuroglia cells and their subcellular compartments, organelles. Cytoplasmic processes and their remodeling interactions also might occur in LC/PASC [
92].
Importantly, Bernard-Valnet et al. [
93] just shared (June 2021) their results from 22 patients with acute COVID-19. They examined the cerebrospinal fluid (CSF) for chemokines and concluded that their results did not indicate an active replication of SARS-CoV-2 in the CSF or massive inflammation in the CSF compartment; however, they did highlight a specific impairment of the neurovascular unit which authors felt were linked to intrathecal production of the chemokine CXCL 8. Importantly, they found that the CSF was characterized by an elevated protein and elevated albumin ratio indicative of blood–brain barrier dysfunction and leakage. Furthermore, they suggested that encephalopathies in severe SARS-CoV-2–infected patients are not due to a major viral infection of the brain. However, they did identify a strong link between encephalopathies and brain barrier impairments. This suggested that one potential mechanism could be the activation of neurovascular cells (endothelial cells, pericytes, astrocytes, and ependymal cells) which could lead to barrier disruption due to a combination of peripheral inflammation (
PCC) and/or hypoxia. Further, these authors suggested a role for corticosteroid usage as a result of their findings [
93].
SARS-CoV-2 has a definite brain neurotropism via its ability to bind to the angiotensin-converting enzyme 2 (ACE2) receptor on the cell membranes of neurovascular unit brain endothelial cells, neurons (both excitatory and inhibitory), and neuroglia, which include the microglia cells, astrocytes, and oligodendrocytes [
94,
95,
96]. Additionally, hyper-activation of the immune system and an excessive aberrant cytokine storm with excessive amounts of
PCC in an attempt to eradicate the SARS-CoV-2 which causes COVID-19 may trigger autoimmune responses and autoimmune disease. Multiple factors have been linked to autoimmune responses and disease, which includes genetics, age, environment, as well as viral infections including COVID-19 and now the emerging LC/PASC post-viral syndrome [
97]. Autoimmunity induced by SARS-CoV-2 tropism to both peripheral organs and the central nervous system are both possible. Certain mechanisms are currently thought to be involved and include molecular mimicry between viral SARS-CoV-2 and host self-antigen epitopes, breakdown of immune tolerance (possibly due to excessive fatigue, and even burnout of the adaptive immune systems due high sustained viral loads, which result in lymphopenia and an increase in the neutrophil/lymphocyte ratio), non-specific bystander activation, epitope spreading, super-antigen presentation, and even stimulation of inflammasome platforms [
94,
95,
96,
97,
98]. Once SARS-CoV-2 enters the cell via its S-1 spike protein, it will hijack the host cells organelles, nucleus, and its reproductive machinery to generate as many progeny virions as it can, while leaving the infected host cell disabled or damaged significantly and may even cause the host cell to die along with liberating a great amount of fragmented host cell self-antigens (Ag). Due to this invasion by SARS-CoV-2 and the response to injury response, there will be an influx of neutrophils initially followed by macrophages and dendritic cells of the innate immune system with even a greater amount of collateral damage to normal, non-infected local regional cells. Regardless, a high viral load will result in greater viral invasion and a greater amount of damaged fragmented molecules, and subsequent fragmented self-antigens will be liberated. These fragmented self-antigens and viral peptide antigens will set the stage for autoimmunity. Furthermore, the major histocompatibility complex (
MHC)positive antigen presenting cells will present these viral antigens to the acquired immune system’s auto-reactive T-cells that will form the antibody which will then develop the antigen–antibody complex [
94,
99]. These possibilities should cause health care providers to become more vigilant in pursuing symptoms and complaints that may be related to the possibility of an increased emergence of autoimmune diseases as the future unfolds in the LC/PASC post-viral syndrome. In LC/PASC, the stage has been set for the development of autoimmunity, and this area of research is ripe in the knowledge to be gained. A first example of this would be the application of testing LC/PASC for autoantibodies [
100] and compare those to the group who have had documented COVID-19 and recovered without developing LC/PASC.
In summary, LC/PASC is a multifactorial post-COVID-19 syndrome associated with multiple pathobiological etiologies which extend well beyond the usual time for healing and recovery. This syndrome has many parallels to myalgic encephalomyelitis/chronic fatigue syndrome with fatigue—lethargy, neurological, and neuropsychiatric symptoms. Multiple ultrastructural brain remodeling changes may be associated with this post-viral syndrome in a response to injury mechanism, as discussed in this review. Neuroglia activation and cellular remodeling, as demonstrated in the stress injury, and the response to injury wound healing mechanisms of diabetic mice models, as outlined in this review, may be similar to those who develop LC/PASC. Therefore, neuroglial activation due to increased PCC and CNSCC along with neuroinflammation might define the neurological outcomes to help explain why some post-acute COVID-19 patients develop LC/PASC syndrome due to viral infections caused by SARS-CoV-2 during this COVID-19 pandemic.
Like streams of dust emanating from a comet, LC/PASC appears to have a lasting effect from acute COVID-19 infections, and this long tail of COVID-19 is reminiscent of the long lasting 1997 comet Hale-Bopp. Unfortunately, we will continue to see the long tail of COVID-19 and will continue to observe it for some time. However, it is hoped that COVID-19 will eventually dissipate and disappear; however, we must also be prepared to co-exist with coronavirus, similar to how we have co-existed with the influenzae virus. References [
101,
102,
103] are cited in the
Supplementary Materials.