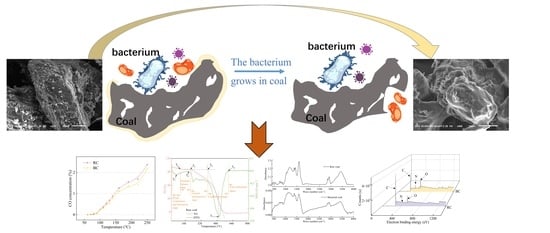
Study on the Influence of Coal Structure and Oxidation Performance by Endogenous Bacterium
Abstract
:1. Introduction
2. Experimental Design and Method
2.1. Culture, Identification, and Morphological Characteristics of Coal Endogenous Bacterium
2.1.1. Isolation of Coal Endogenous Bacterium
2.1.2. Growth Morphology and Curve of Coal Endogenous Bacterium
2.1.3. Molecular Biological Identification of the Bacterium
2.2. Coal Sample Preparation
2.3. Oxidizing Properties of Coal after Bacterial Reaction
2.3.1. Programmed Heating Experiment
2.3.2. Thermal Simultaneous Analysis Equipment and Parameter
2.4. Analysis of Microscopic Group Changes in Bacterial Action
2.4.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.4.2. X-ray Photoelectron Spectroscopy (XPS)
3. Results and Discussion
3.1. Coal Spontaneous Combustion Index Gas CO
3.2. TG–DTG Curve Analysis
3.3. Differential Scanning Calorimetry (DSC) Curve Analysis
3.4. FTIR Fitting Analysis
3.5. X-ray Photoelectron Spectral Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pan, R.K.; Fu, D.; Xiao, Z.J.; Chen, L. The inducement of coal spontaneous combustion disaster and control technology in a wide range of coal mine closed area. Environ. Earth Sci. 2018, 77, 375. [Google Scholar] [CrossRef]
- He, M. Research on Combustion State and Gas Analysis of Sealed Fire Zone in Underground Coal Mine. Ph.D. Thesis, China University of Mining & Technology, Beijing, China, 2013. [Google Scholar]
- Zhang, C.; Ti, Z.; Li, Z.X. Simulation analysis of residual coal temperature prediction based on air leakage volume of goaf. J. Disaster Prev. Mitig. Eng. 2015, 35, 328–332+424. [Google Scholar]
- Tang, Y.B.; Wang, J.F.; Xue, S.; Wu, J.M.; Yu, D.Y. Variation in spontaneous combustion properties of bituminous under inert environment of underground fire zone. J. China Coal Soc. 2016, 41, 439–443. [Google Scholar]
- Ding, X. Numerical Simulation of Microthermal Nitrogen Injection for Fire Prevention and Extinguishing in Gob. Master’s Thesis, China University of Mining & Technology, Xuzhou, China, 2014. [Google Scholar]
- Zhang, J. Treatment of Coal Spontaneous Combustion Fire in Drifting Tunnel Passing through goaf. Min. Saf. Environ. Prot. 2012, 39, 63–65. [Google Scholar]
- Lu, X.; Zhu, H.; Wang, D.; Hu, C.; Zhao, H.; Huo, Y. Flow characteristic investigation of inhibition foam used for fire extinguishment in the underground goaf. Trans. Inst. Chem. Eng. Part B 2018, 116, 159–168. [Google Scholar] [CrossRef]
- Jiang, F.; Sun, W.; Li, Z.; Liang, R.; Li, L.; Liang, Q. Stage inhibition characteristics of composite inhibitor in process of inhibiting coal spontaneous combustion. Coal Sci. Technol. 2022, 50, 68–75. [Google Scholar]
- Zhang, Y.; Shi, X.; Li, Y.Q.; Liu, Y.; Li, S.S.; Huang, Y. Mechanism and inhibiting effects of environmental-friendly inhibitor on coal spontaneous combustion. J. China Univ. Min. Technol. 2018, 47, 1224–1232. [Google Scholar]
- Kang, H.L.; Liu, X.R.; Zhang, Y.W.; Zhao, S.S.; Zhou, A.N. Bacteria solubilization of Shenmu lignite: Influence of surfactants and characterization of the biosolubilization products. Energy Sources Part A Recovery Util. Environ. Eff. 2021, 43, 1162–1180. [Google Scholar] [CrossRef]
- Kang, H.L.; Liu, X.R.; Zhao, S.S.; Yang, Z.W.; Yang, Z. Study on Degradation of Chifeng Lignite of Inner Mongolia by Four Kinds of Bacteria. Coal Technol. 2019, 38, 130–133. [Google Scholar]
- Yi, X.; Zhang, S.H.; Ge, L.; Bai, Z.J.; Deng, J. Present situation and prospect of research on mechanism of inhibiting coal spontaneous combustion by aerobic microorganisms. Clean Coal Technol. 2023, 29, 198–205. [Google Scholar]
- Yang, Y.; Liu, J.; Liu, F.; Wang, Z.; Zhang, Z. Comprehensive evolution mechanism of SOx formation during pyrite oxidation. Proc. Combust. Inst. 2019, 37, 2809–2819. [Google Scholar] [CrossRef]
- Huggins, F.E.; Huffman, G.P.; Zhou, L.X. Low temperature oxidation of bituminous coal: Its detection and effect on coal conversion. Coal Convers. 1987, 15, 233–244. [Google Scholar] [CrossRef]
- Kiani, M.H.; Ahmadi, A.; Zilouei, H. Biological removal of sulphur and ash from fine-grained high pyritic sulphur coals using a mixed culture of mesophilic microorganisms. Fuel 2014, 131, 89–95. [Google Scholar] [CrossRef]
- Cardona, I.C.; Márquez, M.A. Biodesulfurization of two Colombian coals with native microorganisms. Fuel Process. Technol. 2009, 90, 1099–1106. [Google Scholar] [CrossRef]
- Wang, R.; Liu, Z.Y.; Wang, Q.; Peng, C.N.; Chen, Z.H.; Li, F.L.; Wang, X.L.; Yang, C. Experimental on CO2-assisted aerobic-bacteria coal desulfurization process. Chem. Ind. Eng. Prog. 2021, 40, 526–533. [Google Scholar]
- Wang, L. Screening of Microorganisms from Lignite and Surface Modification to Fine Coals by Rhodotorula Mucilaginosa. Ph.D. Thesis, China University of Mining & Technology, Beijing, China, 2012. [Google Scholar]
- Yang, X.; Lai, J.L.; Li, J.; Zhang, Y.; Luo, X.G.; Han, M.W.; Zhao, S.P. Biodegradation and physiological response mechanism of Bacillus aryabhattai to cyclotetramethylenete-tranitramine (HMX) contamination. J. Environ. Manag. 2021, 288, 112247. [Google Scholar] [CrossRef]
- Nakatani, A.S.; Martines, A.M.; Nogueira, M.A.; Fagotti, D.S.; Oliveira, A.G.; Bini, D.; Cardoso, E.J.B.N. Changes in the genetic structure of Bacteria and microbial activity in an agricultural soil amended with tannery sludge. Soil Biol. Biochem. 2011, 43, 106–114. [Google Scholar] [CrossRef]
- Min, W.; Yu, Z.; Zhang, H. Molecular characterization of microbial communities in bioaerosols of a coal mine by 454 pyrosequencing and real-time PCR. J. Environ. Sci. 2015, 30, 241–251. [Google Scholar]
- Kulakova, A.N.; Stafford, T.M.; Larkin, M.J.; Kulakov, L.A. Plasmid pRTL1 Controlling 1-Chloroalkane Degradation by Rhodococcus rhodochrous NCIMB13064. Plasmid 1995, 33, 208–217. [Google Scholar] [CrossRef]
- Zou, G.G.; She, J.; Peng, S.P.; Yin, Q.C.; Liu, H.B.; Che, Y.Y. Two-dimensional SEM image-based analysis of coal porosity and its pore structure. Int. J. Coal Sci. Technol. 2020, 7, 350–361. [Google Scholar] [CrossRef]
- Zhao, L.; Guan, H.N.; Hui, W.; Qian, S.; Gang, W.; Bing, Y.J.; Chao, Z. Molecular structure characterization of lignite treated with ionic liquid via FTIR and XRD spectroscopy. Fuel 2020, 272, 117705. [Google Scholar] [CrossRef]
- Liang, Y.T.; Tian, F.C.; Guo, B.L.; Liu, Z.D. Experimental investigation on microstructure evolution and spontaneous combustion properties of aerobic heated coal. Fuel 2021, 306, 121766. [Google Scholar] [CrossRef]
- Hong, C.; Yang, Q.; Wang, Z.Q.; Xing, Y.; Shen, Q.; Li, Y.F.; Jia, M.M.; Feng, L.H. Co-combustion characteristics and kinetic analysis of antibiotic bacterial residue and coal. CIESC J. 2017, 68, 360–368. [Google Scholar]
- Lü, H.F.; Deng, J.; Li, D.J.; Xu, F.; Xiao, Y.; Shu, C.M. Effect of oxidation temperature and oxygen concentration on macro characteristics of pre-oxidised coal spontaneous combustion process. Energy 2021, 227, 120431. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Mastalerz, M.; Schimmelmann, A. Characterization of chemical functional groups in macerals across different coal ranks via micro-FTIR spectroscopy. Int. J. Coal Geol. 2012, 104, 22–33. [Google Scholar] [CrossRef]
- Ma, R.J.; Zhang, S.; Hou, D.D.; Liu, W.Y.; Yuan, L.; Liu, Q.F. Model construction and optimization of molecule structure of high-rank coal in Feng County, Shaanxi Province. J. China Coal Soc. 2019, 44, 1827–1835. [Google Scholar]
- Machnikowska, H.; Krztoń, A.; Machnikowski, J. The characterization of coal macerals by diffuse reflectance infrared spectroscopy. Fuel 2002, 81, 245–252. [Google Scholar] [CrossRef]
- Zhang, D.K.; Li, Y.H.; Cang, L.P.; Zi, C.Y.; Zhang, Y.Q.; Tian, G.C.; Zhao, W.B. Construction of the Molecular Structure Model of the Zhaotong Lignite Using Simulation and Experiment Data. Chin. J. Struct. Chem. 2022, 41, 137–147. [Google Scholar]
- Jing, Z.; Rodrigues, S.; Strounina, E.; Li, M.; Wood, B.; Underschultz, J.R.; Steel, K.M. Use of FTIR, XPS, NMR to characterize oxidative effects of NaClO on coal molecular structures. Int. J. Coal Geol. 2019, 201, 1–13. [Google Scholar] [CrossRef]
- Lv, J.H.; Wei, X.Y.; Zhang, Y.Y.; Zong, Z.M. Structural characterization of Baiyinhua lignite via direct and thermal de-composition methods. Fuel 2019, 253, 1042–1047. [Google Scholar] [CrossRef]
- Li, K.; Liu, Q.F.; Hou, D.D.; Wang, Z.G.; Zhang, S. Quantitative investigation on the structural characteristics and evolution of high-rank coals from Xinhua, Hunan Province, China. Fuel 2021, 289, 119945. [Google Scholar] [CrossRef]

















| Name | Main Component | Function |
|---|---|---|
| Luria–Bertani (LB) liquid medium | Tryptone, yeast extract, NaCl, deionized water | It enables intensive microbial growth |
| Luria–Bertani (LB) solid medium | Tryptone, yeast extract, NaCl, agar powder, deionized water | It purifies and enriches the target strain |
| Luria–Bertani (LB) solid–coal medium | 200 mesh or more coal powder, tryptone, yeast extract, NaCl, agar powder, deionized water | It verifies whether coal affects the growth of the target strain |
| Coal Sample | Cdaf (%) | Hdaf (%) | Odaf (%) | Ndaf (%) | St,d (%) 1 |
|---|---|---|---|---|---|
| Raw coal (RC) | 76.58 | 4.60 | 14.51 | 1.00 | 0.30 |
| Bacterial coal (BC) | 71.82 | 4.41 | 15.42 | 1.37 | 0.28 |
| Coal Sample | T1 | T2 | T4 | T5 | T6 | T7 | T8 | |
|---|---|---|---|---|---|---|---|---|
| Raw coal (RC) | Temperature (°C) | 73.91 | 111.12 | 193.31 | 216.04 | 386.81 | 496.63 | 596.04 |
| Mass (%) | 98.28 | 96.54 | 95.19 | 94.89 | 71.47 | 25.44 | 2.85 | |
| DTG (%·min−1) | −0.0562 | −0.0399 | −0.0064 | −0.0253 | −0.1694 | −0.7299 | −0.0004 | |
| Bacterial coal (BC) | Temperature (°C) | 84.22 | 132.14 | 208.78 | 295.51 | 400.23 | 483.93 | 624.55 |
| Mass (%) | 97.40 | 95.09 | 94.58 | 93.19 | 81.86 | 38.85 | 5.37 | |
| DTG (%·min−1) | −0.0735 | −0.0243 | −0.0113 | −0.0361 | −0.2532 | −0.5787 | −0.0152 | |
| Coal Sample | TS0 | Tf0 | T4 | T5 | T6 | TS1 | TS2 | T8 | |
|---|---|---|---|---|---|---|---|---|---|
| Raw coal (RC) | Temperature (°C) | 36.12 | 117.88 | 193.31 | 216.04 | 386.81 | 500.88 | 323.52 | 596.05 |
| DSC (mW·mg−1) | −0.0076 | 0.0318 | 0.1950 | 0.2854 | 1.0796 | 3.1551 | 1.0006 | 0.5177 | |
| Integral (J/g) | −0.05 | −2.83 | −0.56 | 2.58 | 110.18 | 298.74 | 57.85 | 411.40 | |
| Bacterial coal (BC) | Temperature (°C) | 56.98 | 129.83 | 208.78 | 295.51 | 400.23 | 532.90 | 324.34 | 624.55 |
| DSC (mW·mg−1) | −0.0328 | 0.0044 | 0.1032 | 0.4323 | 0.9961 | 2.8360 | 0.5426 | 0.5156 | |
| Integral (J/g) | −0.90 | −6.84 | −13.91 | −11.74 | 22.75 | 239.81 | −5.32 | 303.61 | |
| Wave Number (cm−1) | Chemical Bond or Functional Group | Peak Area Percentage (%) | Wave Number (cm−1) | Chemical BOND or Functional Group | Peak Area Percentage (%) | ||
|---|---|---|---|---|---|---|---|
| RC | BC | RC | BC | ||||
| 700~730 | Mono-substitution | 1.7 | 4.0 | 2800~2850 | Methylene symmetric stretching vibration | 26.9 | 25.4 |
| 730~770 | Ortho-substitution | 34.3 | 24.4 | 2850~2890 | Methyl symmetric expansion vibration | 18.5 | 19.0 |
| 770~810 | Meta-substitution | 15.3 | 23.4 | 2900~2950 | Methylene anti-symmetric stretching vibration | 39.2 | 39.9 |
| 810~860 | 1,3,5 Tri-substitution | 26.2 | 27.7 | 2950~3000 | Methyl anti-symmetric telescopic vibration | 15.5 | 15.6 |
| 860~900 | Penta-substitution | 22.3 | 20.5 | 3000~3100 | Hydrogen bonds formed by hydroxyl and N atoms | 4.4 | 4.8 |
| 1000~1250 | C-O stretching vibration | 27.2 | 24.2 | 3100~3200 | Hydrogen bonds formed by cyclic tightly bonded hydroxyl groups | 12.5 | 13.1 |
| 1260~1350 | In-plane bending vibration in primary alcohol-OH | 12.4 | 13.3 | 3200~3300 | Hydrogen bond formed by hydroxyl group and ether oxygen | 17.0 | 20.8 |
| 1350~1410 | In-plane bending vibration in tertiary alcohol-OH | 9.4 | 10.7 | 3300~3400 | Hydrogen bonds formed by self-associative hydroxyl groups | 27.8 | 33.8 |
| 1410~1450 | Aromatic acid bending vibration in the hydroxyl plane | 11.0 | 10.2 | 3400~3500 | Hydrogen bonds formed by hydroxyl groups and π-bonds | 24.9 | 23.2 |
| 1450~1620 | Thick ring C=C skeleton expansion vibration | 29.1 | 29.8 | 3500~3600 | Free hydroxyl group | 13.4 | 4.3 |
| 1650~1740 | Aliphatic acid C=O stretching vibration | 11.0 | 11.7 | ||||
| Elements | Characteristic Groups for Peak Attribution | (RC) Peak Position/eV | (BC) Peak Position/eV | (RC) Relative Content/% | (BC) Relative Content/% |
|---|---|---|---|---|---|
| C1s | Aromatic structures and alkane substitution groups | 282.81 | 283.14 | 32.15 | 37.03 |
| Phenolic or ether carbon | 283.25 | 283.72 | 30.87 | 30.00 | |
| Carbonyl carbon (C=O) | 283.72 | 285.05 | 18.65 | 26.67 | |
| Carboxy carbon | 284.29 | 287 | 18.33 | 6.30 | |
| O1s | Carbonyl oxygen (C=O) | 530.18 | 530.19 | 29.33 | 39.48 |
| Carbon–oxygen single bond oxygen (C-O) | 531.29 | 531.06 | 44.44 | 42.92 | |
| Carboxyl oxygen | 532.25 | 532.02 | 26.22 | 17.59 | |
| N1s | Nitrogen oxide (N-X) | 400.03 | 400.03 | 6.07 | 8.76 |
| Protonated pyridine nitrogen (N-Q) | 398.94 | 399.3 | 23.83 | 27.65 | |
| Pyrrolizidine (N-5) | 398.3 | 398.75 | 46.73 | 46.08 | |
| Pyridine nitrogen (N-6) | 396.93 | 398.13 | 23.36 | 17.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Wang, F.; Guo, L.; Han, T. Study on the Influence of Coal Structure and Oxidation Performance by Endogenous Bacterium. Fire 2023, 6, 339. https://doi.org/10.3390/fire6090339
Dong X, Wang F, Guo L, Han T. Study on the Influence of Coal Structure and Oxidation Performance by Endogenous Bacterium. Fire. 2023; 6(9):339. https://doi.org/10.3390/fire6090339
Chicago/Turabian StyleDong, Xuanmeng, Fusheng Wang, Liwen Guo, and Tiesheng Han. 2023. "Study on the Influence of Coal Structure and Oxidation Performance by Endogenous Bacterium" Fire 6, no. 9: 339. https://doi.org/10.3390/fire6090339