Microhardness Distribution of Long Magnesium Block Processed through Powder Metallurgy
Abstract
:1. Introduction
2. Materials and Methods
3. Theoretical Considerations and Experimental Results
3.1. Theoretical Considerations
3.2. Sample Microstructures
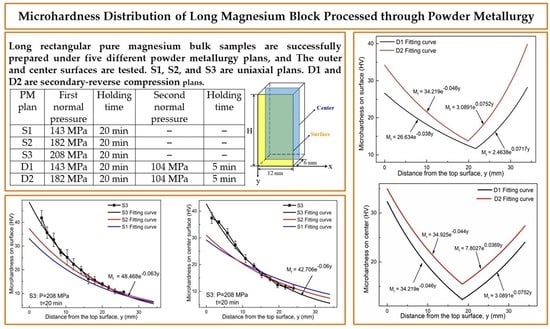
3.3. Hardness Distribution Test Results and Discussion

4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zou, N.; Li, Q. Mechanical Properties of Lightweight Porous Magnesium Processed Through Powder Metallurgy. JOM 2018, 70, 650–655. [Google Scholar] [CrossRef]
- Mondal, D.; Majumder, J.D.; Jha, N.; Badkul, A.; Das, S.; Patel, A.; Gupta, G. Titanium-cenosphere syntactic foam made through powder metallurgy route. Mater. Des. 2012, 34, 82–89. [Google Scholar] [CrossRef]
- Axinte, D.; Andrews, P.; Li, W.; Gindy, N.; Withers, P.; Childs, T. Turning of advanced Ni based alloys obtained via powder metallurgy route. CIRP Ann. 2006, 55, 117–120. [Google Scholar] [CrossRef]
- Danninger, H.; Calderon, R.D.O.; Gierl-Mayer, C. Powder Metallurgy and Sintered Materials. Addit. Manuf. 2017, 19, 4. [Google Scholar] [CrossRef]
- Wu, J.; Guo, R.; Xu, L.; Lu, Z.; Cui, Y.; Yang, R. Effect of Hot Isostatic Pressing Loading Route on Microstructure and Mechanical Properties of Powder Metallurgy Ti2AlNb Alloys. J. Mater. Sci. Technol. 2017, 33, 172–178. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y. Buckling analysis of thin rectangular plates under uniaxial or biaxial compressive point loads by the differential quadrature method. Int. J. Mech. Sci. 2015, 101–102, 38–48. [Google Scholar] [CrossRef]
- Bolzoni, L.; Ruiz-Navas, E.M.; Gordo, E. Quantifying the properties of low-cost powder metallurgy titanium alloys. Mater. Sci. Eng. A 2017, 687, 47–53. [Google Scholar] [CrossRef]
- Bolzoni, L.; Ruiz-Navas, E.M.; Gordo, E. Understanding the properties of low-cost iron-containing powder metallurgy titanium alloys. Mater. Des. 2016, 110, 317–323. [Google Scholar] [CrossRef]
- Ivasishin, O.M.; Anokhin, V.M.; Demidik, A.N.; Savvakin, D.G. Cost-Effective Blended Elemental Powder Metallurgy of Titanium Alloys for Transportation Application. In Key Engineering Materials, 1st ed.; Froes, F.H., Evangelista, E., Eds.; Trans Tech Publications: Zürich, Switzerland, 2000; Volume 188, pp. 55–62. [Google Scholar] [CrossRef]
- Ramakrishnan, P. Automotive applications of powder metallurgy. In Advances in Powder Metallurgy; Elsevier: Amsterdam, The Netherlands, 2013; pp. 493–519. [Google Scholar] [CrossRef]
- Vasconcellos, L.M.R.d.; Oliveira, M.V.d.; Graça, M.L.d.A.; Vasconcellos, L.G.O.d.; Carvalho, Y.R.; Cairo, C.A.A. Porous titanium scaffolds produced by powder metallurgy for biomedical applications. Mater. Res. 2008, 11, 275–280. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Cao, H.; Kang, Y.; Yu, K.; Xiao, T.; Luo, J.; Deng, Y.; Fang, H.; Xiong, H.; Dai, Y. Effects of Zn concentration and heat treatment on the microstructure, mechanical properties and corrosion behavior of as-extruded Mg-Zn alloys produced by powder metallurgy. J. Alloy. Compd. 2017, 693, 1277–1289. [Google Scholar] [CrossRef]
- Khalajabadi, S.Z.; Kadir MR, A.; Izman, S.; Yusop MZ, M. Facile fabrication of hydrophobic surfaces on mechanically alloyed-Mg/HA/TiO2/MgO bionanocomposites. Appl. Surf. Sci. 2015, 324, 380–392. [Google Scholar] [CrossRef]
- Khalajabadi, S.Z.; Kadir, M.R.A.; Izman, S.; Marvibaigi, M. The effect of MgO on the biodegradation, physical properties and biocompatibility of a Mg/HA/MgO nanocomposite manufactured by powder metallurgy method. J. Alloy. Compd. 2016, 655, 266–280. [Google Scholar] [CrossRef]
- Soltani, M.; Karamian, E.; Karimian, M. Survey of AZ31/HA-zeolite nano crystalline biocomposite with powder metallurgy (PM). MPMP 2016, 5, 5–11. [Google Scholar]
- Vogiatzis, C.; Tsouknidas, A.; Kountouras, D.; Skolianos, S. Aluminum–ceramic cenospheres syntactic foams produced by powder metallurgy route. Mater. Des. 2015, 85, 444–454. [Google Scholar] [CrossRef]
- Pournaderi, S.; Akhlaghi, F. Wear behaviour of Al6061-Al2O3 composites produced by in-situ powder metallurgy (IPM). Powder Technol. 2017, 313, 184–190. [Google Scholar] [CrossRef]
- Reddy, T.H.; Pal, S.; Kumar, K.C.; Mohan, M.K.; Kokol, V. Finite Element Analysis for Mechanical Response of Magnesium Foams with Regular Structure Obtained by Powder Metallurgy Method. Procedia Eng. 2016, 149, 425–430. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Lee, S.; Kang, S.; Jeon, J.; Lee, S.H.; Kim, H.-K.; Choi, H. Complex effects of alloy composition and porosity on the phase transformations and mechanical properties of powder metallurgy steels. Powder Technol. 2015, 284, 459–466. [Google Scholar] [CrossRef]
- Ravichandran, M.; Anandakrishnan, V. Optimization of powder metallurgy parameters to attain maximum strength coefficient in Al–10 wt% MoO3 composite. J. Mater. Res. 2015, 30, 2380–2387. [Google Scholar] [CrossRef]
- Liang, Y.; Yang, F.; Zhang, L.; Lin, J.; Shang, S.; Liu, Z.-K. Reaction behavior and pore formation mechanism of TiAl–Nb porous alloys prepared by elemental powder metallurgy. Intermetallics 2014, 44, 1–7. [Google Scholar] [CrossRef]
- Kwon, H.; Mondal, J.; AlOgab, K.A.; Sammelselg, V.; Takamichi, M.; Kawaski, A.; Leparoux, M. Graphene oxide-reinforced aluminum alloy matrix composite materials fabricated by powder metallurgy. J. Alloy. Compd. 2017, 698, 807–813. [Google Scholar] [CrossRef]
- Cristofolini, I.; Molinari, A.; Pederzini, G.; Rambelli, A. Study of the uniaxial cold compaction of AISI 316L stainless steel powders through single action tests. Powder Technol. 2016, 295, 284–295. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, L.; Lin, J.; Liang, Y.; He, Y.; Shang, S.; Liu, Z.-K. Pore structure and gas permeability of high Nb-containing TiAl porous alloys by elemental powder metallurgy for microfiltration application. Intermetallics 2013, 33, 2–7. [Google Scholar] [CrossRef]
- Li, B.; Yan, F.; Lu, X. Effect of microstructure on the tensile property of porous Ti produced by powder metallurgy technique. Mater. Sci. Eng. A 2012, 534, 43–52. [Google Scholar] [CrossRef]
- Vajpai, S.; Dube, R.; Sangal, S. Application of rapid solidification powder metallurgy processing to prepare Cu–Al–Ni high temperature shape memory alloy strips with high strength and high ductility. Mater. Sci. Eng. A 2013, 570, 32–42. [Google Scholar] [CrossRef]
- Hamidi, A.G.; Arabi, H.; Rastegari, S. A feasibility study of W-Cu composites production by high pressure compression of tungsten powder. Int. J. Refract. Met. Hard Mater. 2011, 29, 123–127. [Google Scholar] [CrossRef]
- Liu, J.; Khan, U.; Coleman, J.; Fernandez, B.; Rodriguez, P.; Naher, S.; Brabazon, D. Graphene oxide and graphene nanosheet reinforced aluminium matrix composites: Powder synthesis and prepared composite characteristics. Mater. Des. 2016, 94, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.; Kang, S.; Choi, Y.; Choi, H.; Lee, S.-J. Effect of relative density on microstructure and mechanical properties of Fe-12Mn-0.2C alloy fabricated by powder metallurgy. Powder Technol. 2016, 298, 106–111. [Google Scholar] [CrossRef]
- Čapek, J.; Vojtěch, D. Properties of porous magnesium prepared by powder metallurgy. Mater. Sci. Eng. C 2013, 33, 564–569. [Google Scholar] [CrossRef]
- Jha, N.; Mondal, D.; Majumdar, J.D.; Badkul, A.; Jha, A.; Khare, A. Highly porous open cell Ti-foam using NaCl as temporary space holder through powder metallurgy route. Mater. Des. 2013, 47, 810–819. [Google Scholar] [CrossRef]
- Mohammed, M.T.; Hussein, H.A.; Jasim, I.N.; Allawi, Z.I. Effect of compaction pressure on morphology and physical properties for Cu-based produce by using powder metallurgy technique. In Proceedings of the 2018 1st International Scientific Conference of Engineering Sciences-3rd Scientific Conference of Engineering Science (ISCES), Diyala, Iraq, 10–11 January 2018. [Google Scholar]
- Rajković, V.; Božić, D.; Popović, M.; Jovanović, M. Properties of Cu-Al2O3 powder and compact composites of various starting particle size obtained by high-energy milling. AMES MJOM 2009, 15, 45–52. [Google Scholar]
- Zou, N.; Li, Q. Microstructure and hardness of porous magnesium processed by powder metallurgy using polystyrene as the space holder. In Magnesium Technology 2020, 1st ed.; Jordon, J., Miller, V., Joshi, V., Neelameggham, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 387–391. [Google Scholar]
- Zou, N.; Li, Q. Effect of Compaction Pressure and Magnesium Weight Fraction on Hardness of Recycled-Polystyrene Matrix Composite. JOM 2018, 70, 1454–1458. [Google Scholar] [CrossRef]
- Briscoe, B.; Evans, P. Wall friction in the compaction of agglomerated ceramic powders. Powder Technol. 1991, 65, 7–20. [Google Scholar] [CrossRef]
- St-Laurent, S.; Chagnon, F.; Thomas, Y. Study of compaction and ejection properties of powder mixes processed by warm compaction. Adv. Powder Metall. Part. Mater. 2000, 1, 3–79. [Google Scholar]
- Cante Terán, J.C.; Oliver Olivella, X.; González Ferrari, C.; Riera Colom, M.D.; Istúriz, Á.; Prado Pozuelo, J.M. Experimental and numerical study of the die filling stage in powder metallurgy. In Proceedings of the European Powder Metallurgy 2005, Prague, Czech Republic, 2–5 October 2005. [Google Scholar]
- Wu, C.-Y.; Ruddy, O.; Bentham, A.; Hancock, B.; Best, S.; Elliott, J. Modelling the mechanical behaviour of pharmaceutical powders during compaction. Powder Technol. 2005, 152, 107–117. [Google Scholar] [CrossRef]
- Jiang, L.; Li, Z.; Fan, G.; Cao, L.; Zhang, D. The use of flake powder metallurgy to produce carbon nanotube (CNT)/aluminum composites with a homogenous CNT distribution. Carbon 2012, 50, 1993–1998. [Google Scholar] [CrossRef]
- Strijbos, S.; Rankin, P.; Wassink, R.K.; Bannink, J.; Oudemans, G. Stresses occurring during one-sided die compaction of powders. Powder Technol. 1977, 18, 187–200. [Google Scholar] [CrossRef]
- Li, W.-H.; Ding, K.; Tian, H.-R.; Yao, M.-S.; Nath, B.; Deng, W.-H.; Wang, Y.; Xu, G. Conductive Metal-Organic Framework Nanowire Array Electrodes for High-Performance Solid-State Supercapacitors. Adv. Funct. Mater. 2017, 27, 1702067. [Google Scholar] [CrossRef]
- Fang, Z.Z.; Paramore, J.D.; Sun, P.; Chandran, K.R.; Zhang, Y.; Xia, Y.; Cao, F.; Koopman, M.; Free, M. Powder metallurgy of titanium–past, present, and future. Int. Mater. Rev. 2018, 63, 407–459. [Google Scholar] [CrossRef] [Green Version]
- Li, X.Q.; Jiang, Y.M.; Peng, Z.; Fu, L.P.; Zuo, J.; Zheng, H.P.; Wang, L.Z. Janssen behavior of silos with Rankine passive stresses. J. Shandong Univ. Nat. Sci. 2010, 45, 101–104. [Google Scholar]
- Horne, R.; Nedderman, R. Analysis of the stress distribution in two-dimensional bins by the method of characteristics. Powder Technol. 1976, 14, 93–102. [Google Scholar] [CrossRef]
- Janssen, H. Versuche uber getreidedruck in silozellen. Z. Ver. Dtsch. Ing. 1895, 39, 1045–1049. [Google Scholar]
- Walker, D. An approximate theory for pressures and arching in hoppers. Chem. Eng. Sci. 1966, 21, 975–997. [Google Scholar] [CrossRef]
- Walters, J. A theoretical analysis of stresses in silos with vertical walls. Chem. Eng. Sci. 1973, 28, 13–21. [Google Scholar] [CrossRef]
- Walters, J. A theoretical analysis of stresses in axially-symmetric hoppers and bunkers. Chem. Eng. Sci. 1973, 28, 779–789. [Google Scholar] [CrossRef]
- Shang, C.; Sinka, I.C.; Pan, J. Constitutive Model Calibration for Powder Compaction Using Instrumented Die Testing. Exp. Mech. 2012, 52, 903–916. [Google Scholar] [CrossRef]
- Güner, F.; Cora, Ö.N.; Sofuoğlu, H. Effects of friction models on the compaction behavior of copper powder. Tribol. Int. 2018, 122, 125–132. [Google Scholar] [CrossRef]
- Cameron, I.M.; Gethin, D.T. Exploration of die wall friction for powder compaction using a discrete finite element modelling technique. Model. Simul. Mater. Sci. Eng. 2001, 9, 289–307. [Google Scholar] [CrossRef]
- Siyayisa, C.; Van Rooyen, G.; Stumpf, W. Metallurgical factors that affect the strand width during continuous casting of DIN 1.4003 stainless steel. JSAIMM 2005, 105, 473–481. [Google Scholar]
- Hentschel, M.L.; Page, N.W. Elastic properties of powders during compaction. Part 1: Pseudo-isotropic moduli. J. Mater. Sci. 2007, 42, 1261–1268. [Google Scholar] [CrossRef]
- Sinka, I.; Cunningham, J.; Zavaliangos, A. The effect of wall friction in the compaction of pharmaceutical tablets with curved faces: A validation study of the Drucker–Prager Cap model. Powder Technol. 2003, 133, 33–43. [Google Scholar] [CrossRef]
- Kim, W.-J.; Chung, S.; Chung, C.; Kum, D. Superplasticity in thin magnesium alloy sheets and deformation mechanism maps for magnesium alloys at elevated temperatures. Acta Mater. 2001, 49, 3337–3345. [Google Scholar] [CrossRef]
- Somekawa, H.; Mukai, T. Effect of grain refinement on fracture toughness in extruded pure magnesium. Scr. Mater. 2005, 53, 1059–1064. [Google Scholar] [CrossRef]
- Ishikawa, K.; Watanabe, H.; Mukai, T. High temperature compressive properties over a wide range of strain rates in an AZ31 magnesium alloy. J. Mater. Sci. 2005, 40, 1577–1582. [Google Scholar] [CrossRef]
- Han, B.; Dunand, D. Microstructure and mechanical properties of magnesium containing high volume fractions of yttria dispersoids. Mater. Sci. Eng. A 2000, 277, 297–304. [Google Scholar] [CrossRef]
- Long, W.M. Radial pressures in powder compaction. Powder Met. 1960, 3, 73–86. [Google Scholar] [CrossRef]
- Wiącek, J.; Stasiak, M.; Parafiniuk, P. Effective elastic properties and pressure distribution in bidisperse granular packings: DEM simulations and experiment. Arch. Civ. Mech. Eng. 2017, 17, 271–280. [Google Scholar] [CrossRef]
- Khoei, A.; Keshavarz, S.; Khaloo, A. Modeling of large deformation frictional contact in powder compaction processes. Appl. Math. Model. 2008, 32, 775–801. [Google Scholar] [CrossRef]
- Modnet, P. Measurement of friction for powder compaction modelling–comparison between laboratories. Powder Metall. 2000, 43, 364–374. [Google Scholar]
- Guyoncourt, D.; Tweed, J.; Gough, A.; Dawson, J.; Pater, L. Constitutive data and friction measurements of powders using instrumented die. Powder Met. 2001, 44, 25–33. [Google Scholar] [CrossRef]
- Cameron, I.M.; Gethin, D.T.; Tweed, J.H. Friction measurement in powder die compaction by shear plate technique. Powder Met. 2002, 45, 345–353. [Google Scholar] [CrossRef]
- Ooia, T.; Yusofa, Y.; Taliba, R.; Othmana, S.; Aziza, M. Changes in friction, surface roughness, and tensile strength upon compaction of Ficus deltoidea powder. ScienceAsia 2013, 39, 520–526. [Google Scholar] [CrossRef] [Green Version]
- Fitriani, P.; Kwon, H.; Han, Y.S.; Lee, S.-M.; Yoon, D.-H. Granule rearrangement and pore structure of a spray-dried alumina compact observed by X-ray tomography. J. Eur. Ceram. Soc. 2020, 40, 2445–2452. [Google Scholar] [CrossRef]
- Chen, W.; Yamamoto, Y.; Peter, W.H.; Clark, M.B.; Nunn, S.D.; Kiggans, J.; Muth, T.R.; Blue, C.A.; Williams, J.C.; Akhtar, K. The investigation of die-pressing and sintering behavior of ITP CP-Ti and Ti-6Al-4V powders. J. Alloy. Compd. 2012, 541, 440–447. [Google Scholar] [CrossRef]
- Singh, A.; Singh, J.; Sinha, M.K.; Kumar, R.; Verma, V. Compaction and Densification Characteristics of Iron Powder/Coal Fly Ash Mixtures Processed by Powder Metallurgy Technique. J. Mater. Eng. Perform. 2021, 30, 1207–1220. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, P.M. Development of Mg based biomaterial with improved mechanical and degradation properties using powder metallurgy. J. Magnes. Alloy. 2020, 8, 883–898. [Google Scholar] [CrossRef]
- Sebastian, K.; Melnikov, A.; Sivagurunathan, K.; Guo, X.; Wang, X.; Mandelis, A. Non-destructive lock-in thermography of green powder metallurgy component inhomogeneities: A predictive imaging method for manufactured component flaw prevention. NDT E Int. 2022, 127, 102603. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, D.; Hwang, J.; Ryu, H.J.; Hong, S.H. Fabrication and characterization of powder metallurgy tantalum components prepared by high compaction pressure technique. Mater. Charact. 2016, 114, 225–233. [Google Scholar] [CrossRef]
- Ghorbani, A.; Sheibani, S.; Ataie, A. Microstructure and mechanical properties of consolidated Cu-Cr-CNT nanocomposite prepared via powder metallurgy. J. Alloy. Compd. 2018, 732, 818–827. [Google Scholar] [CrossRef]
- Kumar, N.; Bharti, A.; Saxena, K.K. A re-investigation: Effect of powder metallurgy parameters on the physical and mechanical properties of aluminium matrix composites. Mater. Today Proc. 2021, 44, 2188–2193. [Google Scholar] [CrossRef]
- Amaranan, S.; Manonukul, A. Study of process parameters in conventional powder metallurgy of silver. J. Met. Mater. Miner 2010, 20, 51–55. [Google Scholar]
- Kumar, N.; Bharti, A.; Saxena, K.K. A re-analysis of effect of various process parameters on the mechanical properties of Mg based MMCs fabricated by powder metallurgy technique. Mater. Today Proc. 2020, 26, 1953–1959. [Google Scholar] [CrossRef]








| Material | Pressure (MPa) | Size (mm) | H/Ø | Reference |
|---|---|---|---|---|
| Mg/HA/TiO2/MgO | 840 | 5: Ø 12 | 0.42 | [13] |
| Mg/HA/MgO | 840 | 5: Ø 12 | 0.42 | [14] |
| AZ31/HA-Zeolite | 1000 | 20: Ø 12 | 1.67 | [15] |
| Al-Ceramic | 200; 250; 300 | 20: Ø 12 | 1.67 | [16] |
| Al6061-Al2O3 | 200–800 | 10: Ø 25 | 0.40 | [17] |
| Porous Mg Monoliths | 265 | 16: Ø 13 | 1.23 | [18] |
| Carbon Steels | 300–1250 | 9.5: Ø 8 | 1.19 | [19] |
| Al-10wt% MoO3 Composite | 250; 300; 350 | 12: Ø 24 | 0.50 | [20] |
| Ti-48Al-6Nb | 300 | 2: Ø 10 | 0.20 | [21] |
| Graphene Oxide-Reinforced Al Alloy | 570 | 5: Ø 30 | 0.17 | [22] |
| Mg-3Al-1Zn Alloy | 550 | 20: Ø 82 | 0.24 | [23] |
| Nb-Ti-Al Porous Alloys | 300 | 3: Ø 32 | 0.094 | [24] |
| Ti and Polymethyl Methacrylate | 500 | 20 × 20 × 2.5 | 0.13 | [25] |
| Cu-Al-Ni | 500 | 30 × 18 × 6 | 0.33 | [26] |
| W Powder with Fisher Sub Sieve | 200–663 | 20: Ø 20 | 1.00 | [27] |
| rGO/GNS-AMC Nanocomposites | 30; 73; 220; 260; 330; 560 | 1: Ø 20 | 0.050 | [28] |
| Fe-12Mn-0.2C Alloy | 250; 500; 900 | 10: Ø 12 | 0.83 | [29] |
| Mg Powder and NH₄HCO₃ Powder | 265 | 30: Ø 16 | 1.88 | [30] |
| Ti-Powder and NaCl Crystals | 200 | 50: Ø 20 | 2.50 | [31] |
| PM Plan | First Normal Pressure | Holding Time | Second Normal Pressure | Holding Time |
|---|---|---|---|---|
| S1 | 143 MPa | 20 min | – | – |
| S2 | 182 MPa | 20 min | – | – |
| S3 | 208 MPa | 20 min | – | – |
| D1 | 143 MPa | 20 min | 104 MPa | 5 min |
| D2 | 182 MPa | 20 min | 104 MPa | 5 min |
| PM Plan | Surface Hardness (HV) | Center Hardness (HV) | ||||
|---|---|---|---|---|---|---|
| Top | Middle | Bottom | Top | Middle | Bottom | |
| S1 | 30.4 ± 2.6 | 12.9 ± 0.5 | 8.9 ± 1.5 | 29.9 ± 0.7 | 12.5 ± 1.9 | 11.7 ± 2.0 |
| S2 | 34.0 ± 2.8 | 15.7 ± 1.0 | 9.2 ± 1.5 | 29.1 ± 0.7 | 14.9 ± 1.9 | 10.4 ± 2.3 |
| S3 | 41.9 ± 3.1 | 16.2 ± 2.1 | 10.5 ± 0.6 | 37.4 ± 2.0 | 15.4 ± 0.5 | 11.2 ± 1.1 |
| D1 | 24.2 ± 1.7 | 12.4 ± 3.2 | 16.7 ± 4.5 | 29.4 ± 1.9 | 13.7 ± 1.5 | 18.7 ± 3.3 |
| D2 | 32.0 ± 4.1 | 14.9 ± 3.2 | 21.4 ± 8.6 | 31.5 ± 1.6 | 15.5 ± 0.7 | 21.2 ± 1.7 |
| Sample | Surface Hardness Fitting Curve Equation | (y = 0) | (y = 34) | Center Hardness Fitting Curve Equation | (y = 0) | (y = 34) |
|---|---|---|---|---|---|---|
| S1−1 | 33.91 | 7.34 | 29.98 | 10.81 | ||
| S1−2 | 30.11 | 6.30 | 27.31 | 8.89 | ||
| S1−3 | 33.77 | 5.96 | 29.64 | 7.35 | ||
| S2−1 | 40.38 | 6.66 | 28.37 | 7.53 | ||
| S2−2 | 40.55 | 6.25 | 31.32 | 6.34 | ||
| S2−3 | 29.81 | 7.15 | 32.37 | 9.20 | ||
| S3−1 | 48.47 | 5.69 | 42.71 | 5.55 |
| Sample | Surface Hardness Fitting Curve Equation | Center Hardness Fitting Curve Equation |
|---|---|---|
| D1−1 (h = 20.53) | ||
| D1−2 (h = 14.67) | ||
| D1−3 (h = 21.5) | ||
| D2−1 (h = 19.52) | ||
| D2−2 (h = 19.78) | ||
| D2−3 (h = 20.36) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Li, Q. Microhardness Distribution of Long Magnesium Block Processed through Powder Metallurgy. J. Manuf. Mater. Process. 2023, 7, 5. https://doi.org/10.3390/jmmp7010005
Wang J, Li Q. Microhardness Distribution of Long Magnesium Block Processed through Powder Metallurgy. Journal of Manufacturing and Materials Processing. 2023; 7(1):5. https://doi.org/10.3390/jmmp7010005
Chicago/Turabian StyleWang, Jiaying, and Qizhen Li. 2023. "Microhardness Distribution of Long Magnesium Block Processed through Powder Metallurgy" Journal of Manufacturing and Materials Processing 7, no. 1: 5. https://doi.org/10.3390/jmmp7010005