A Versatile Setup for Measuring Multiple Behavior Endpoints in Zebrafish
Abstract
:1. Introduction
2. Material and Methods
2.1. Animals and Housing
2.2. Ethanol Treatment
2.3. Video-Tracking and Data Analysis
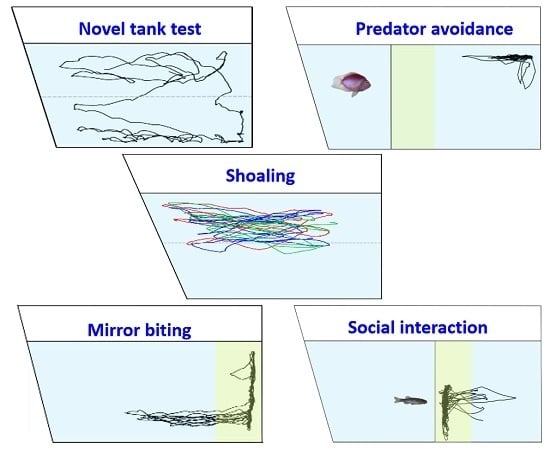
3. Results
4. Discussion
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Dooley, K.; Zon, L.I. Zebrafish: A model system for the study of human disease. Curr. Opin. Genet. Dev. 2000, 10, 252–256. [Google Scholar] [CrossRef]
- Speedie, N.; Gerlai, R. Alarm substance induced behavioral responses in zebrafish (Danio rerio). Behav. Brain Res. 2008, 188, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Pagnussat, N.; Piato, A.L.; Schaefer, I.C.; Blank, M.; Tamborski, A.R.; Guerim, L.D.; Bonan, C.D.; Vianna, M.R.; Lara, D.R. One for all and all for one: The importance of shoaling on behavioral and stress responses in zebrafish. Zebrafish 2013, 10, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Gerlai, R.; Lee, V.; Blaser, R. Effects of acute and chronic ethanol exposure on the behavior of adult zebrafish (Danio rerio). Pharmacol. Biochem. Behav. 2006, 85, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Egan, R.J.; Bergner, C.L.; Hart, P.C.; Cachat, J.M.; Canavello, P.R.; Elegante, M.F.; Elkhayat, S.I.; Bartels, B.K.; Tien, A.K.; Tien, D.H. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav. Brain Res. 2009, 205, 38–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbazuk, W.B.; Korf, I.; Kadavi, C.; Heyen, J.; Tate, S.; Wun, E.; Bedell, J.A.; McPherson, J.D.; Johnson, S.L. The syntenic relationship of the zebrafish and human genomes. Genome Res. 2000, 10, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Lieschke, G.J.; Currie, P.D. Animal models of human disease: Zebrafish swim into view. Nat. Rev. Genet. 2007, 8, 353. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Stewart, A.M.; Gerlai, R. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol. Sci. 2014, 35, 63–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bambino, K.; Chu, J. Zebrafish in toxicology and environmental health. Curr. Top Dev. Biol. 2017, 124, 331–367. [Google Scholar] [PubMed]
- Dai, Y.J.; Jia, Y.F.; Chen, N.; Bian, W.P.; Li, Q.K.; Ma, Y.B.; Chen, Y.L.; Pei, D.S. Zebrafish as a model system to study toxicology. Environ. Toxicol. Chem. 2014, 33, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Teraoka, H.; Dong, W.; Hiraga, T. Zebrafish as a novel experimental model for developmental toxicology. Congenit. Anomal. 2003, 43, 123–132. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Gebhardt, M.; Stewart, A.M.; Cachat, J.M.; Brimmer, M.; Chawla, J.S.; Craddock, C.; Kyzar, E.J.; Roth, A.; Landsman, S. Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish 2013, 10, 70–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaser, R.; Chadwick, L.; McGinnis, G. Behavioral measures of anxiety in zebrafish (Danio rerio). Behav. Brain Res. 2010, 208, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.T.; MacRae, C.A. Systematic approaches to toxicology in the zebrafish. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 433–453. [Google Scholar] [CrossRef] [PubMed]
- Gerlai, R. Social behavior of zebrafish: From synthetic images to biological mechanisms of shoaling. J. Neurosci. Methods 2014, 234, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Strungaru, S.-A.; Robea, M.A.; Plavan, G.; Todirascu-Ciornea, E.; Ciobica, A.; Nicoara, M. Acute exposure to methylmercury chloride induces fast changes in swimming performance, cognitive processes and oxidative stress of zebrafish (Danio rerio) as reference model for fish community. J. Trace Element. Med. Biol. 2018, 47, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Cachat, J.; Stewart, A.; Grossman, L.; Gaikwad, S.; Kadri, F.; Chung, K.M.; Wu, N.; Wong, K.; Roy, S.; Suciu, C. Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nature Protoc. 2010, 5, 1786. [Google Scholar] [CrossRef] [PubMed]
- Cachat, J.; Stewart, A.; Utterback, E.; Hart, P.; Gaikwad, S.; Wong, K.; Kyzar, E.; Wu, N.; Kalueff, A.V. Three-dimensional neurophenotyping of adult zebrafish behavior. PLoS ONE 2011, 6, e17597. [Google Scholar] [CrossRef] [PubMed]
- Blaser, R.; Gerlai, R. Behavioral phenotyping in zebrafish: Comparison of three behavioral quantification methods. Behav. Res. Methods 2006, 38, 456–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bencan, Z.; Sledge, D.; Levin, E.D. Buspirone, chlordiazepoxide and diazepam effects in a zebrafish model of anxiety. Pharmacol. Biochem. Behav. 2009, 94, 75–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, M.; Raymond, J.; Hester, J.; Kyzar, E.; Gaikwad, S.; Bruce, I.; Fryar, C.; Chanin, S.; Enriquez, J.; Bagawandoss, S. Assessing social behavior phenotypes in adult zebrafish: Shoaling, social preference, and mirror biting tests. In Zebrafish Protocols for Neurobehavioral Research; Springer: Berlin, Germany, 2012; pp. 231–246. [Google Scholar]
- Engeszer, R.E.; da Barbiano, L.A.; Ryan, M.J.; Parichy, D.M. Timing and plasticity of shoaling behaviour in the zebrafish, danio rerio. Anim. Behav. 2007, 74, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Saverino, C.; Gerlai, R. The social zebrafish: Behavioral responses to conspecific, heterospecific, and computer animated fish. Behav. Brain Res. 2008, 191, 77–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moretz, J.A.; Martins, E.P.; Robison, B.D. The effects of early and adult social environment on zebrafish (Danio rerio) behavior. Environ.Biol. Fishes 2007, 80, 91–101. [Google Scholar] [CrossRef]
- Gerlai, R.; Fernandes, Y.; Pereira, T. Zebrafish (Danio rerio) responds to the animated image of a predator: Towards the development of an automated aversive task. Behav. Brain Res. 2009, 201, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Bass, S.L.; Gerlai, R. Zebrafish (Danio rerio) responds differentially to stimulus fish: The effects of sympatric and allopatric predators and harmless fish. Behav. Brain Res. 2008, 186, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Cachat, J.; Kyzar, E.J.; Collins, C.; Gaikwad, S.; Green, J.; Roth, A.; El-Ounsi, M.; Davis, A.; Pham, M.; Landsman, S. Unique and potent effects of acute ibogaine on zebrafish: The developing utility of novel aquatic models for hallucinogenic drug research. Behav. Brain Res. 2013, 236, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Toms, C.N.; Echevarria, D.J. Back to basics: Searching for a comprehensive framework for exploring individual differences in zebrafish (Danio rerio) behavior. Zebrafish 2014, 11, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Puttonen, H.A.; Sundvik, M.; Rozov, S.; Chen, Y.-C.; Panula, P. Acute ethanol treatment upregulates th1, th2, and hdc in larval zebrafish in stable networks. Front. Neural Circ. 2013, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Escudero, A.; Vicente-Page, J.; Hinz, R.C.; Arganda, S.; De Polavieja, G.G. Idtracker: Tracking individuals in a group by automatic identification of unmarked animals. Nat. Methods 2014, 11, 743. [Google Scholar] [CrossRef] [PubMed]
- Audira, G.; Sampurna, B.P.; Juniardi, S.; Liang, S.-T.; Lai, Y.-H.; Hsiao, C.-D. A simple setup to perform 3D locomotion tracking in zebrafish by using a single camera. Inventions 2018, 3, 11. [Google Scholar] [CrossRef]
- Ali, S.; Champagne, D.L.; Alia, A.; Richardson, M.K. Large-scale analysis of acute ethanol exposure in zebrafish development: A critical time window and resilience. PLoS ONE 2011, 6, e20037. [Google Scholar] [CrossRef] [PubMed]
- Dlugos, C.A.; Rabin, R.A. Ethanol effects on three strains of zebrafish: Model system for genetic investigations. Pharmacol. Biochem. Behav. 2003, 74, 471–480. [Google Scholar] [CrossRef]
- Avdesh, A.; Chen, M.; Martin-Iverson, M.T.; Mondal, A.; Ong, D.; Rainey-Smith, S.; Taddei, K.; Lardelli, M.; Groth, D.M.; Verdile, G. Regular care and maintenance of a zebrafish (Danio rerio) laboratory: An introduction. J. Visual. Exp. 2012. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.C.; Anthony, C.D. Using randomization techniques to analyse behavioural data. Anim. Behav. 1996, 51, 733–738. [Google Scholar] [CrossRef]
- Blaser, R.; Penalosa, Y. Stimuli affecting zebrafish (Danio rerio) behavior in the light/dark preference test. Physiol. Behav. 2011, 104, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, B.; Bjerke, S.; Kobayashi, K.; Guo, S. Acute effects of alcohol on larval zebrafish: A genetic system for large-scale screening. Pharmacol. Biochem. Behav. 2004, 77, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Cachat, J.; Canavello, P.; Elegante, M.; Bartels, B.; Hart, P.; Bergner, C.; Egan, R.; Duncan, A.; Tien, D.; Chung, A. Modeling withdrawal syndrome in zebrafish. Behav. Brain Res. 2010, 208, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Gerlai, R. Zebra fish: An uncharted behavior genetic model. Behav. Genet. 2003, 33, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.M.; Nguyen, M.; Wong, K.; Poudel, M.K.; Kalueff, A.V. Developing zebrafish models of autism spectrum disorder (ASD). Progr. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 50, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Norton, W.; Bally-Cuif, L. Adult zebrafish as a model organism for behavioural genetics. BMC Neurosci. 2010, 11, 90. [Google Scholar] [CrossRef] [PubMed]
- Spence, R.; Gerlach, G.; Lawrence, C.; Smith, C. The behaviour and ecology of the zebrafish, Danio rerio. Biol. Rev. 2008, 83, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Seguin, D.; Shams, S.; Gerlai, R. Behavioral responses to novelty or to a predator stimulus are not altered in adult zebrafish by early embryonic alcohol exposure. Alcohol. Clin. Exp. Res. 2016, 40, 2667–2675. [Google Scholar] [CrossRef] [PubMed]
- Gerlai, R.; Lahav, M.; Guo, S.; Rosenthal, A. Drinks like a fish: Zebra fish (Danio rerio) as a behavior genetic model to study alcohol effects. Pharmacol. Biochem. Behav. 2000, 67, 773–782. [Google Scholar] [CrossRef]
- Guo, S. Linking genes to brain, behavior and neurological diseases: What can we learn from zebrafish? Genes, Brain Behav. 2004, 3, 63–74. [Google Scholar] [CrossRef]
- Fernandes, Y.; Gerlai, R. Long-term behavioral changes in response to early developmental exposure to ethanol in zebrafish. Alcohol. Clin. Exp. Res. 2009, 33, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Pather, S.; Gerlai, R. Shuttle box learning in zebrafish (Danio rerio). Behav. Brain Res. 2009, 196, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.O.; Annan, L.V.; Kanellopoulos, A.H.; Brock, A.J.; Combe, F.J.; Baiamonte, M.; Teh, M.-T.; Brennan, C.H. The utility of zebrafish to study the mechanisms by which ethanol affects social behavior and anxiety during early brain development. Progr. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 55, 94–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidel, A.J.; Assmann, K.L.; Werlang, C.C.; Bertoncello, K.T.; Francescon, F.; Rambo, C.L.; Beltrame, G.M.; Calegari, D.; Batista, C.B.; Blaser, R.E. Subchronic atrazine exposure changes defensive behaviour profile and disrupts brain acetylcholinesterase activity of zebrafish. Neurotoxicol. Teratol. 2014, 44, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.; Gerlai, R. Quantification of shoaling behaviour in zebrafish (Danio rerio). Behav. Brain Res. 2007, 184, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Buske, C.; Gerlai, R. Early embryonic ethanol exposure impairs shoaling and the dopaminergic and serotoninergic systems in adult zebrafish. Neurotoxicol. Teratol. 2011, 33, 698–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousas, C.; Newbury, P.; Anagnostopoulos, C.-N. Measuring the Steps: Generating Action Transitions between Locomotion Behaviours. In Proceedings of the 18th International Conference on Computer Games: AI, Animation, Mobile, Interactive Multimedia, Educational & Serious Games (CGAMES), Louisville, KT, USA, 30 July–1 August 2013; pp. 31–35. [Google Scholar]
- Durupınar, F. From Audiences to Mobs: Crowd Simulation with Psychological Factors. Ph.D. Thesis, Bilkent University, Çankaya, Üniversiteler, Turkey, 2010. [Google Scholar]
- Van Basten, B.J.; Jansen, S.E.; Karamouzas, I. Exploiting motion capture to enhance avoidance behaviour in games. In Proceedings of the International Workshop on Motion in Games, Zeist, The Netherlands, 21–24 November 2009; pp. 29–40. [Google Scholar]
- Braun, A.; Musse, S.R.; de Oliveira, L.P.L.; Bodmann, B.E. Modeling individual behaviors in crowd simulation. In Proceedings of the 16th International Conference on Computer Animation and Social Agents, New Brunswick, NJ, USA, 7–9 May 2003; pp. 143–148. [Google Scholar]
- Brooks, S.P.; Dunnett, S.B. Tests to assess motor phenotype in mice: A user’s guide. Nat. Rev. Neuroscience 2009, 10, 519. [Google Scholar] [CrossRef] [PubMed]
- Nabeshima, T.; Kim, H.-C. Involvement of genetic and environmental factors in the onset of depression. Experim. Neurobiol. 2013, 22, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Brigman, J.L.; Graybeal, C.; Holmes, A. Predictably irrational: Assaying cognitive inflexibility in mouse models of schizophrenia. Front. Neurosci. 2010, 4, 13. [Google Scholar] [CrossRef] [PubMed]




| Behavior Endpoints (units) | Definition | Applicable to | Data Interpretation |
|---|---|---|---|
| Average speed (cm·s−1) | Total distance traveled divided by total time duration | Novel tank test, mirror-biting test, predator test, social interaction test, shoaling test | Reflects general motor/neurological phenotypes |
| Maximum speed (cm·s−1) | Maximum speed fish capable to reach | ||
| Reflects motor aspects of zebrafish swimming, may be increased or decreased depending on the nature of behavioral test | |||
| Minimum speed (cm·s−1) | Minimum speed fish capable to reach | ||
| Distance traveled (cm) | Total distance the zebrafish traveled within the novel tank | ||
| Freezing time percentage (%) | Total percentage of time when speed less than 1 cm·s−1 | ||
| Indicates increased anxiety and freezing time is generally higher in stressed zebrafish | |||
| Swimming time percentage (%) | Total percentage of time when speed 1–10 cm·s−1 | ||
| Rapid time movement percentage (%) | Total percentage of time when speed more than 10 cm·s−1 | ||
| Time spent in top percentage (%) | Total time spent in the top portion of the novel tank | Novel tank test, predator test, shoaling test | Increasing value indicates lower anxiety levels |
| Time spent in top/bottom ratio | Total time spent in the top and bottom portion of the novel tank ratio | ||
| Distance traveled in the top (cm) | Total distance traveled in the top portion of the novel tank | ||
| Distance traveled top/bottom ratio | Total distance traveled in the top and bottom portion of the novel tank ratio | ||
| Number of entries to the top | Total times zebrafish swims to the upper half of the tank | Novel tank test | |
| Average entry duration (s) | Time spent in top divided by the number of entries to the top | ||
| Latency to enter top (s) | The amount of time it takes the fish to cross into the upper half of the tank | Increasing value indicates higher anxiety levels | |
| Mirror biting time percentage (%) | Total percentage of time when zebrafish bit the mirror | Mirror-biting test | Increasing value indicates higher aggression levels |
| Longest duration in mirror side percentage (%) | Total percentage of zebrafish longest duration stayed in front of mirror | ||
| Predator approaching time percentage (%) | Total percentage of time when zebrafish approached the predator | Predator test | Decreasing value indicates higher anxiety levels |
| Average distance to predator separator (cm) | Average distance of zebrafish to separator for predator | Increasing value indicates higher anxiety levels | |
| Zebrafish interaction time percentage (%) | Total percentage of time when zebrafish interacted with another zebrafish | Social interaction test | Increasing value indicates higher sociability levels |
| Longest duration in separator side percentage (%) | Total percentage of zebrafish longest duration stayed in front of separator | ||
| Average distance to zebrafish separator (cm) | Average distance of zebrafish to separator for another zebrafish | Decreasing value indicates higher sociability levels | |
| Average inter-fish distance (cm) | Average distance between the body center of every member of the shoal | Shoaling test | Increasing value indicates lower anxiety levels |
| Average shoal area (cm2) | Average size of the shoal | ||
| Thigmotaxis (cm) | The average distance of the group from the center of the tank | Decreasing value indicates lower anxiety levels | |
| Average nearest neighbor distance (cm) | Distance for the body center of each fish to the closest neighboring fish | Increasing value indicates lower anxiety levels | |
| Average farthest neighbor distance (cm) | Distance for the body center of each fish to the farthest neighboring fish |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Audira, G.; Sampurna, B.P.; Juniardi, S.; Liang, S.-T.; Lai, Y.-H.; Hsiao, C.-D. A Versatile Setup for Measuring Multiple Behavior Endpoints in Zebrafish. Inventions 2018, 3, 75. https://doi.org/10.3390/inventions3040075
Audira G, Sampurna BP, Juniardi S, Liang S-T, Lai Y-H, Hsiao C-D. A Versatile Setup for Measuring Multiple Behavior Endpoints in Zebrafish. Inventions. 2018; 3(4):75. https://doi.org/10.3390/inventions3040075
Chicago/Turabian StyleAudira, Gilbert, Bonifasius Putera Sampurna, Stevhen Juniardi, Sung-Tzu Liang, Yu-Heng Lai, and Chung-Der Hsiao. 2018. "A Versatile Setup for Measuring Multiple Behavior Endpoints in Zebrafish" Inventions 3, no. 4: 75. https://doi.org/10.3390/inventions3040075