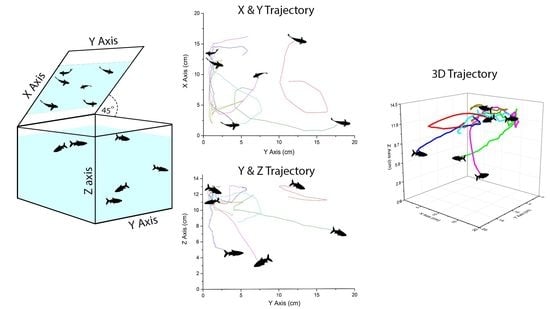
A Simple Setup to Perform 3D Locomotion Tracking in Zebrafish by Using a Single Camera
Abstract
:- First, execute “idTracker.exe” and open the selected video for analysis.
- “Number of individuals” must be set to the number of zebrafish used.
- Set the “Intensity threshold”. For this parameter, the system is going to consider the pixels with a lower intensity than this threshold as the animals (vice versa, if “Invert contrast” is checked).
- “Minimum size” of the fish must be determined. The blobs will be rejected if they are smaller than the minimum size entered.
- If the sizes of the animals are larger than 2000 pixels, a number higher than 1 must be input in the “Resolution reduction” (the number of pixels will be divided by n2, where n is the number in the box).
- To activate the background removal option, “Remove background” and click “Comp were selected Bckgrnd” button to compute before the tracking process starts.
- If only part of the video will be tracked, the “Interval” box must be selected.
- The number of reference frames must be set. A lower number should be chosen for increased speed, and a higher number should be chosen for increased accuracy.
- The Region of Interest (ROI) and/or exclude regions can also be adjusted by pressing on the “Include region”, “Exclude region”, and “Clear” buttons.
- The number of processors also can be set by filling a number in the “# of processors” box, indicating to how many processors idTracker will be applied (‘Inf’ means that idTracker will be used for all available processors).
- Finally, the analysis will be started by pressing the “Start” button. If “S&E” (Save & Exit) button is selected, the program will end without starting the tracking. All tracking parameters can be used later if the “Load previous data” button is selected.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lieschke, G.J.; Currie, P.D. Animal models of human disease: Zebrafish swim into view. Nat. Rev. Genet. 2007, 8, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Parng, C.; Seng, W.L.; Semino, C.; McGrath, P. Zebrafish: A preclinical model for drug screening. Assay Drug Dev. Technol. 2002, 1, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Champagne, D.L.; Spaink, H.P.; Richardson, M.K. Zebrafish embryos and larvae: A new generation of disease models and drug screens. Birth Defects Res. Part C Embryo Today Rev. 2011, 93, 115–133. [Google Scholar] [CrossRef] [PubMed]
- Lessman, C.A. The developing zebrafish (Danio rerio): A vertebrate model for high-throughput screening of chemical libraries. Birth Defects Res. Part C Embryo Today Rev. 2011, 93, 268–280. [Google Scholar] [CrossRef] [PubMed]
- MacPhail, R.; Brooks, J.; Hunter, D.; Padnos, B.; Irons, T.; Padilla, S. Locomotion in larval zebrafish: Influence of time of day, lighting and ethanol. Neurotoxicology 2009, 30, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Miklósi, Á.; Andrew, R.J. The zebrafish as a model for behavioral studies. Zebrafish 2006, 3, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Stewart, A.M.; Gerlai, R. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol. Sci. 2014, 35, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Fetcho, J.R.; Liu, K.S. Zebrafish as a model system for studying neuronal circuits and behavior. Ann. N. Y. Acad. Sci. 1998, 860, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Egan, R.J.; Bergner, C.L.; Hart, P.C.; Cachat, J.M.; Canavello, P.R.; Elegante, M.F.; Elkhayat, S.I.; Bartels, B.K.; Tien, A.K.; Tien, D.H. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav. Brain Res. 2009, 205, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.; Gaikwad, S.; Kyzar, E.; Green, J.; Roth, A.; Kalueff, A.V. Modeling anxiety using adult zebrafish: A conceptual review. Neuropharmacology 2012, 62, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Maximino, C.; de Brito, T.M.; da Silva Batista, A.W.; Herculano, A.M.; Morato, S.; Gouveia, A. Measuring anxiety in zebrafish: A critical review. Behav. Brain Res. 2010, 214, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Blaser, R.; Chadwick, L.; McGinnis, G. Behavioral measures of anxiety in zebrafish (Danio rerio). Behav. Brain Res. 2010, 208, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.; Krause, J. Repeated measures of shoaling tendency in zebrafish (Danio rerio) and other small teleost fishes. Nat. Protoc. 2006, 1, 1828–1831. [Google Scholar] [CrossRef] [PubMed]
- Buske, C.; Gerlai, R. Shoaling develops with age in zebrafish (Danio rerio). Prog. Neuro Psychopharmacol. Biol. Psychiatry 2011, 35, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.; Gerlai, R. Quantification of shoaling behaviour in zebrafish (Danio rerio). Behav. Brain Res. 2007, 184, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Engeszer, R.E.; Ryan, M.J.; Parichy, D.M. Learned social preference in zebrafish. Curr. Biol. 2004, 14, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Scerbina, T.; Chatterjee, D.; Gerlai, R. Dopamine receptor antagonism disrupts social preference in zebrafish: A strain comparison study. Amino Acids 2012, 43, 2059–2072. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.; Maximino, C.; Marques de Brito, T.; Herculano, A.M.; Gouveia, A.; Morato, S.; Cachat, J.M.; Gaikwad, S.; Elegante, M.F.; Hart, P.C. Neurophenotyping of adult zebrafish using the light/dark box paradigm. Zebrafish Neurobehav. Protoc. 2011, 157–167. [Google Scholar]
- Steenbergen, P.J.; Richardson, M.K.; Champagne, D.L. Patterns of avoidance behaviours in the light/dark preference test in young juvenile zebrafish: A pharmacological study. Behav. Brain Res. 2011, 222, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Emran, F.; Rihel, J.; Dowling, J.E. A behavioral assay to measure responsiveness of zebrafish to changes in light intensities. J. Vis. Exp. 2008. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.M.; Gaikwad, S.; Kyzar, E.; Kalueff, A.V. Understanding spatio-temporal strategies of adult zebrafish exploration in the open field test. Brain Res. 2012, 1451, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Richardson, M.K. Exploratory behaviour in the open field test adapted for larval zebrafish: Impact of environmental complexity. Behav. Process. 2013, 92, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Maaswinkel, H.; Zhu, L.; Weng, W. The immediate and the delayed effects of buspirone on zebrafish (Danio rerio) in an open field test: A 3D approach. Behav. Brain Res. 2012, 234, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Blaser, R.E.; Rosemberg, D.B. Measures of anxiety in zebrafish (Danio rerio): Dissociation of black/white preference and novel tank test. PLoS ONE 2012, 7, e36931. [Google Scholar] [CrossRef] [PubMed]
- Maximino, C.; Benzecry, R.; Oliveira, K.R.M.; Batista, E.D.J.O.; Herculano, A.M.; Rosemberg, D.B.; de Oliveira, D.L.; Blaser, R. A comparison of the light/dark and novel tank tests in zebrafish. Behaviour 2012, 149, 1099–1123. [Google Scholar] [CrossRef]
- Blaser, R.; Gerlai, R. Behavioral phenotyping in zebrafish: Comparison of three behavioral quantification methods. Behav. Res. Methods 2006, 38, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Morrow-Tesch, J.; Dailey, J.; Jiang, H. A video data base system for studying animal behavior. J. Anim. Sci. 1998, 76, 2605–2608. [Google Scholar] [CrossRef] [PubMed]
- Cachat, J.M.; Canavello, P.R.; Elegante, M.F.; Bartels, B.K.; Elkhayat, S.I.; Hart, P.C.; Tien, A.K.; Tien, D.H.; Beeson, E.; Mohnot, S. Modeling stress and anxiety in zebrafish. Zebrafish Models Neurobehav. Res. 2011, 73–88. [Google Scholar] [CrossRef]
- Stewart, A.M.; Grieco, F.; Tegelenbosch, R.A.; Kyzar, E.J.; Nguyen, M.; Kaluyeva, A.; Song, C.; Noldus, L.P.; Kalueff, A.V. A novel 3D method of locomotor analysis in adult zebrafish: Implications for automated detection of CNS drug-evoked phenotypes. J. Neurosci. Methods 2015, 255, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Theriault, D.; Wu, Z.; Hristov, N.; Swartz, S.; Breuer, K.; Kunz, T.; Betke, M. Reconstruction and Analysis of 3D Trajectories of Brazilian Free-Tailed Bats in Flight; CS Department, Boston University: Boston, MA, USA, 2010. [Google Scholar]
- Dahmen, H.-J.; Zeil, J. Recording and reconstructing three-dimensional trajectories: A versatile method for the field biologist. Proc. R. Soc. Lond. B Biol. Sci. 1984, 222, 107–113. [Google Scholar] [CrossRef]
- Zhu, L.; Weng, W. Catadioptric stereo-vision system for the real-time monitoring of 3D behavior in aquatic animals. Physiol. Behav. 2007, 91, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Macrì, S.; Neri, D.; Ruberto, T.; Mwaffo, V.; Butail, S.; Porfiri, M. Three-dimensional scoring of zebrafish behavior unveils biological phenomena hidden by two-dimensional analyses. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Cario, C.L.; Farrell, T.C.; Milanese, C.; Burton, E.A. Automated measurement of zebrafish larval movement. J. Physiol. 2011, 589, 3703–3708. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Escudero, A.; Vicente-Page, J.; Hinz, R.C.; Arganda, S.; de Polavieja, G.G. Idtracker: Tracking individuals in a group by automatic identification of unmarked animals. Nat. Methods 2014, 11, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Conklin, E.E.; Lee, K.L.; Schlabach, S.A.; Woods, I.G. Videohacking: Automated tracking and quantification of locomotor behavior with open source software and off-the-shelf video equipment. J. Undergrad. Neurosci. Educ. 2015, 13, A120. [Google Scholar] [PubMed]
- Wang, Y.-N.; Hou, Y.-Y.; Sun, M.-Z.; Zhang, C.-Y.; Bai, G.; Zhao, X.; Feng, X.-Z. Behavioural screening of zebrafish using neuroactive traditional chinese medicine prescriptions and biological targets. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Jackson, B.E.; Evangelista, D.J.; Ray, D.D.; Hedrick, T.L. 3D for the people: Multi-camera motion capture in the field with consumer-grade cameras and open source software. Biol. Open 2016, 5, 1334–1342. [Google Scholar] [CrossRef] [PubMed]
- Cachat, J.M.; Stewart, A.; Utterback, E.; Kyzar, E.; Hart, P.C.; Carlos, D.; Gaikwad, S.; Hook, M.; Rhymes, K.; Kalueff, A.V. Deconstructing adult zebrafish behavior with swim trace visualizations. Zebrafish Neurobehav. Protoc. 2011, 191–201. [Google Scholar] [CrossRef]
- Avdesh, A.; Chen, M.; Martin-Iverson, M.T.; Mondal, A.; Ong, D.; Rainey-Smith, S.; Taddei, K.; Lardelli, M.; Groth, D.M.; Verdile, G. Regular care and maintenance of a zebrafish (Danio rerio) laboratory: An introduction. J. Vis. Exp. 2012. [Google Scholar] [CrossRef] [PubMed]
- Leris, I.; Sfakianakis, D.; Kentouri, M. Are zebrafish danio rerio males better swimmers than females? J. Fish Biol. 2013, 83, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Pavlidis, M.; Digka, N.; Theodoridi, A.; Campo, A.; Barsakis, K.; Skouradakis, G.; Samaras, A.; Tsalafouta, A. Husbandry of zebrafish, Danio rerio, and the cortisol stress response. Zebrafish 2013, 10, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.J.; Zerulla, T.C.; Tierney, K.B. Zebrafish (Danio rerio) as a model for the study of aging and exercise: Physical ability and trainability decrease with age. Exp. Gerontol. 2014, 50, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Cachat, J.M.; Canavello, P.R.; Elkhayat, S.I.; Bartels, B.K.; Hart, P.C.; Elegante, M.F.; Beeson, E.C.; Laffoon, A.L.; Haymore, W.A.; Tien, D.H. Video-aided analysis of zebrafish locomotion and anxiety-related behavioral responses. Zebrafish Neurobehav. Protoc. 2011, 1–14. [Google Scholar] [CrossRef]
- Li, X.; Liu, B.; Li, X.-L.; Li, Y.-X.; Sun, M.-Z.; Chen, D.-Y.; Zhao, X.; Feng, X.-Z. SiO2 nanoparticles change colour preference and cause Parkinson’s-like behaviour in zebrafish. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, X.; Li, T.; Li, X.; Feng, D.; Kuang, X.; Xu, J.; Zhao, X.; Sun, M.; Chen, D. SiO2 nanoparticles cause depression and anxiety-like behavior in adult zebrafish. RSC Adv. 2017, 7, 2953–2963. [Google Scholar] [CrossRef]
- Nema, S.; Hasan, W.; Bhargava, A.; Bhargava, Y. A novel method for automated tracking and quantification of adult zebrafish behaviour during anxiety. J. Neurosci. Methods 2016, 271, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Umeda, K.; Ishizuka, T.; Yawo, H.; Shoji, W. Position-and quantity-dependent responses in zebrafish turning behavior. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with imagej. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Puttonen, H.A.; Sundvik, M.; Rozov, S.; Chen, Y.-C.; Panula, P. Acute ethanol treatment upregulates th1, th2, and hdc in larval zebrafish in stable networks. Front. Neural Circuits 2013, 7. [Google Scholar] [CrossRef] [PubMed]
- Di Chiara, G.; Imperato, A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. USA 1988, 85, 5274–5278. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.L.; Oreschak, K.; Rhinehart, Z.; Robison, B.D. Anxiolytic effects of fluoxetine and nicotine exposure on exploratory behavior in zebrafish. PeerJ 2016, 4, e2352. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.; Chatterjee, D.; Gerlai, R. An integrative analysis of ethanol tolerance and withdrawal in zebrafish (Danio rerio). Behav. Brain Res. 2015, 276, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.; Gerlai, R. Time-course of behavioural changes induced by ethanol in zebrafish (Danio rerio). Behav. Brain Res. 2013, 252, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Maes, J.; Verlooy, L.; Buenafe, O.E.; De Witte, P.A.; Esguerra, C.V.; Crawford, A.D. Evaluation of 14 organic solvents and carriers for screening applications in zebrafish embryos and larvae. PLoS ONE 2012, 7, e43850. [Google Scholar] [CrossRef] [PubMed]
- De Esch, C.; van der Linde, H.; Slieker, R.; Willemsen, R.; Wolterbeek, A.; Woutersen, R.; de Groot, D. Locomotor activity assay in zebrafish larvae: Influence of age, strain and ethanol. Neurotoxicol. Teratol. 2012, 34, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Cachat, J.; Stewart, A.; Utterback, E.; Hart, P.; Gaikwad, S.; Wong, K.; Kyzar, E.; Wu, N.; Kalueff, A.V. Three-dimensional neurophenotyping of adult zebrafish behavior. PLoS ONE 2011, 6, e17597. [Google Scholar] [CrossRef] [PubMed]
- Ladu, F.; Bartolini, T.; Panitz, S.G.; Chiarotti, F.; Butail, S.; Macrì, S.; Porfiri, M. Live predators, robots, and computer-animated images elicit differential avoidance responses in zebrafish. Zebrafish 2015, 12, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Mwaffo, V.; Butail, S.; Porfiri, M. In-silico experiments of zebrafish behaviour: Modeling swimming in three dimensions. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Urtasun, R.; Fleet, D.J.; Fua, P. 3D people tracking with gaussian process dynamical models. In Proceedings of the 2006 IEEE Computer Society Conference on Computer Vision and Pattern Recognition, New York, NY, USA, 17–22 June 2006; pp. 238–245. [Google Scholar]
- Guo, F.; Qian, G. Monocular 3D tracking of articulated human motion in silhouette and pose manifolds. J. Image Video Process. 2008, 2008. [Google Scholar] [CrossRef]
- Li, R.; Tian, T.-P.; Sclaroff, S.; Yang, M.-H. 3D human motion tracking with a coordinated mixture of factor analyzers. Int. J. Comput. Vis. 2010, 87, 170–190. [Google Scholar] [CrossRef]
- Lawrence, N.D. Gaussian process latent variable models for visualisation of high dimensional data. In Advances in Neural Information Processing Systems; MIT Press: Cambridge, MA, USA, 2004; pp. 329–336. [Google Scholar]
- Ji, S.; Xu, W.; Yang, M.; Yu, K. 3D convolutional neural networks for human action recognition. IEEE Trans. Pattern Anal. Mach. Intell. 2013, 35, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Crivellaro, A.; Rad, M.; Verdie, Y.; Moo Yi, K.; Fua, P.; Lepetit, V. A novel representation of parts for accurate 3D object detection and tracking in monocular images. In Proceedings of the IEEE International Conference on Computer Vision, Santiago, Chile, 7–13 December 2015; pp. 4391–4399. [Google Scholar]
- Moreno-Noguer, F. 3D human pose estimation from a single image via distance matrix regression. In Proceedings of the 2017 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Honolulu, HI, USA, 21–26 July 2017; pp. 1561–1570. [Google Scholar]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Audira, G.; Sampurna, B.P.; Juniardi, S.; Liang, S.-T.; Lai, Y.-H.; Hsiao, C.-D. A Simple Setup to Perform 3D Locomotion Tracking in Zebrafish by Using a Single Camera. Inventions 2018, 3, 11. https://doi.org/10.3390/inventions3010011
Audira G, Sampurna BP, Juniardi S, Liang S-T, Lai Y-H, Hsiao C-D. A Simple Setup to Perform 3D Locomotion Tracking in Zebrafish by Using a Single Camera. Inventions. 2018; 3(1):11. https://doi.org/10.3390/inventions3010011
Chicago/Turabian StyleAudira, Gilbert, Bonifasius Putera Sampurna, Stevhen Juniardi, Sung-Tzu Liang, Yu-Heng Lai, and Chung-Der Hsiao. 2018. "A Simple Setup to Perform 3D Locomotion Tracking in Zebrafish by Using a Single Camera" Inventions 3, no. 1: 11. https://doi.org/10.3390/inventions3010011