PbF2–CdF2–SrF2 Ternary Solid Solution: Crystal Growth and Investigation
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
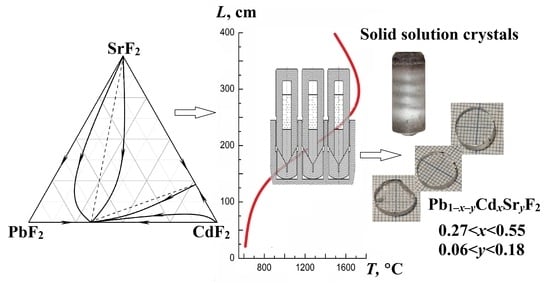
3.1. Analysis of the PbF2–CdF2–SrF2 System—Search for a Region of Homogeneous Compositions
3.2. Optical Properties
3.3. Ionic Conductivity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klimm, D.; Rabe, M.; Bertram, R.; Uecker, R.; Parthier, L. Phase diagram analysis and crystal growth of solid solutions Ca1−xSrxF2. J. Cryst. Growth 2008, 310, 152–155. [Google Scholar] [CrossRef]
- Nafziger, R.H. High-temperature phase transformation in BaF2–SrF2 system. J. Amer. Ceram. Soc. 1971, 54, 467–472. [Google Scholar] [CrossRef]
- Kozak, A.; Samuel, M.; Chretien, A. Miscibilitecristalline des fluorures de calcium, de strontium et de plomb dans le fluorure de cadmium: Fluorure double Cd2BaF6. Rev. Chim. Miner. 1971, 8, 805–811. [Google Scholar]
- O’Horo, M.P.; White, W.B. Phase equilibria in the systems CdF2–CaF2, CdF2–PbF2, and CdF2–ZnF2. J. Am. Ceram. Soc. 1971, 54, 588–589. [Google Scholar] [CrossRef]
- Mouchovski, J.T.; Temelkov, K.A.; Vuchkov, N.K. The growth of mixed alkaline-earth fluorides for laser host applications. Prog. Cryst. Growth Characteriz. Mater. 2011, 57, 1–41. [Google Scholar] [CrossRef]
- Karimov, D.N.; Komarkova, O.N.; Sorokin, N.I.; Bezhanov, V.A.; Chernov, S.P.; Popov, P.A.; Sobolev, B.P. Growth of Congruently Melting Ca0.59Sr0.41F2 Crystals and Study of Their Properties. Crystallogr. Rep. 2010, 55, 518–524. [Google Scholar] [CrossRef]
- Sorokin, N.I.; Karimov, D.N.; Buchinskaya, I.I.; Sobolev, B.P.; Popov, P.A. Electrical and thermal conductivities of congruently melting single crystals of isovalent M1−X M′XF2 solid solutions (M, M′ = Ca, Sr, Cd, Pb) in relation to their defect fluorite structure. Crystallogr. Rep. 2015, 60, 532–536. [Google Scholar] [CrossRef]
- Novikov, V.V.; Matovnikov, A.V.; Mitroshenkov, N.V.; Kuznetsov, S.V. Structural Disorder and Heat Capacity of a Solid Solution between Cadmium and Strontium Fluorides. Inorg. Mater. 2020, 56, 626–632. [Google Scholar] [CrossRef]
- Novikov, V.V.; Mitroshenkov, N.V.; Matovnikov, A.V.; Kuznetsov, S.V. Specific features of lattice dynamics of (CaF2)x(SrF2)(1–x) solid solutions. Mater. Chem. Phys. 2020, 240, 122247. [Google Scholar] [CrossRef]
- Popov, P.A.; Moiseev, N.V.; Karimov, D.N.; Sorokin, N.I.; Sul’yanova, E.A.; Sobolev, B.P.; Konyushkin, V.A.; Fedorov, P.P. Thermophysical characteristics of Ca1−xSrxF2 solid-solution crystals (0 ≤ x ≤ 1). Crystallogr. Rep. 2015, 60, 116–122. [Google Scholar] [CrossRef]
- Popov, P.A.; Krugovykh, A.A.; Konyushkin, V.A.; Nakladov, A.N.; Kuznetsov, S.V.; Fedorov, P.P. Thermal Conductivity of Sr1–xBaxF2 Single Crystals. Inorg. Mater. 2021, 57, 629–633. [Google Scholar] [CrossRef]
- Buchinskaya, I.I.; Fedorov, P.P. A new optical Medium–Cd0.75Sr0.25F2 single crystals. Crystallogr. Rep. 2004, 49, 334–336. [Google Scholar] [CrossRef]
- Karimov, D.N.; Buchinskaya, I.I.; Sorokin, N.I.; Sobolev, B.P.; Popov, P.A. Crystal growth and thermal conductivity of the congruently melting solid solution Cd0.77Sr0.23F2. Inorg. Mater. 2019, 55, 495–499. [Google Scholar] [CrossRef]
- Ushakov, S.N.; Uslamina, M.A.; Pynenkov, A.A.; Mishkin, V.P.; Nishchev, K.N.; Kuznetsov, S.V.; Chernova, E.V.; Fedorov, P.P. Growth and physical properties of CaSrBaF6 single crystals. Condens. Matter Interphases 2021, 23, 93–100. [Google Scholar] [CrossRef]
- Komandin, G.A.; Spector, I.E.; Fedorov, P.P.; Kuznetsov, S.V.; Ushakov, S.N.; Uslamina, M.A.; Nishchev, K.N.; Garibin, E.A. Long-wavelength optical properties of the Ca0.33Sr0.33Ba0.33F2 solid solution single crystals. Opt. Mater. 2022, 127, 112267. [Google Scholar] [CrossRef]
- Basiev, T.T.; Vasil’ev, S.V.; Doroshenko, M.E.; Konuyshkin, V.A.; Kouznetsov, S.V.; Osiko, V.V.; Fedorov, P.P. Efficient lasing in diode-pumping Yb3+:CaF2–SrF2 solid solution single crystals. Quantum Electron. 2007, 37, 934–937. [Google Scholar] [CrossRef]
- Lyapin, A.A.; Ermakov, A.S.; Kuznetsov, S.V.; Gushchin, S.V.; Ryabochkina, P.A.; Konyushkin, V.A.; Nakladov, A.N.; Fedorov, P.P. Upconversion luminescence of CaF2-SrF2-ErF3 single crystals upon 1.5 µm laser excitation. J. Phys. Conf. Ser 2019, 1410, 012086. [Google Scholar] [CrossRef]
- Kuznetsov, S.V.; Konyushkin, V.A.; Nakladov, A.N.; Chernova, E.V.; Fedorov, P.P.; Popov, P.A.; Pynenkov, A.A.; Nishchev, K.N. Thermophysical properties of single crystals of CaF2–SrF2–RF3 (R = Ho, Pr) fluorite solid solutions. Inorg. Mater. 2020, 56, 975–981. [Google Scholar] [CrossRef]
- Ushakov, S.N.; Fedorov, P.P.; Kuznetsov, S.V.; Osiko, V.V.; Uslamina, M.A.; Nishchev, K.N. Study of Yb3+ optical centers in fluoride solid solution crystals CaF2–SrF2–YbF3. Opt. Spectrosc. 2020, 128, 600–604. [Google Scholar] [CrossRef]
- Lyapin, A.A.; Bubnov, M.K.; Bukarev, S.A.; Gushchin, S.V.; Konyushkin, V.A.; Kuznetsov, S.V.; Nakladov, A.N.; Ryabochkina, P.A.; Fedorov, P.P. Up-conversion luminescence of CaF2–SrF2–HoF3 solid solutions upon excitation of the 5I7 level of Ho3+ ions. Opt. Spectrosc. 2023, 131, 346–353. Available online: https://ojs.ioffe.ru/index.php/os/article/view/4085 (accessed on 10 August 2023).
- Sun, Q.M.; Hu, G.Q.; Lin, Z.X.; Yu, L.L.; Liu, X.T.; Yu, Y.S. Preparation and Application of Light Conversion film for exploiting Solar Energy. Adv. Mater. Res. 2013, 827, 44–48. [Google Scholar] [CrossRef]
- Wu, Y.Q.; Su, L.B.; Xu, J.; Chen, H.B.; Li, H.J.; Zheng, L.H.; Wang, Q.G. Spectroscopic and thermal properties of Yb doped CaF2-SrF2 laser crystal. Acta Phys. Sin. 2012, 61, 177801. [Google Scholar] [CrossRef]
- Kosacki, I. Physical properties and applications of Cd1-xPbxF2 superionic crystals. Appl. Phys. A 1989, 49, 413–424. [Google Scholar] [CrossRef]
- Mao, R.; Zhang, L.; Zhu, R.-Y. A Search for Scintillation in Doped Cubic Lead Fluoride Crystals. IEEE Trans. Nucl. Sci. 2010, 57, 3841–3845. [Google Scholar] [CrossRef]
- Lui, M.; McFarlane, R.A.; Yap, D. Growth of Erbium Doped PbF2-SrF2 Epitaxial Layers On GaAs(111)B for Upconversion Waveguide Laser Applications. MRS Online Proc. Libr. 1993, 329, 167–172. [Google Scholar] [CrossRef]
- Weller, P.F. Electrical and Optical Studies of Doped CdF2-CaF2 Crystals. Inorg. Chem. 1966, 5, 736–739. [Google Scholar] [CrossRef]
- Fedorov, P.P.; Buchinskaya, I.I. Spatial inhomogeneity in crystalline materials and saddle-type congruent melting points in ternary systems. Russ. Chem. Rev. 2012, 81, 1. [Google Scholar] [CrossRef]
- US 6649326B2. Photolithographic Method and UV Transmitting Fluoride Crystals with Minimized Spatial Dispersion. Available online: https://patentimages.storage.googleapis.com/19/5d/ec/7f6578c4cb8939/US6649326.pdf (accessed on 7 October 2022).
- Sorokin, N.I.; Buchinskaya, I.I. Ionic Conductivity of Crystals of Fluorite-Type Solid Solution of the PbF2–CdF2–MnF2 System. Crystallogr. Rep. 2022, 67, 958–963. [Google Scholar] [CrossRef]
- Sorokin, N.I. Charge Carrier Mobility in Crystals of the Pb0.67Cd0.33F2 Superionic Conductor. Phys. Solid State 2015, 57, 1352–1356. [Google Scholar] [CrossRef]
- Murin, I.V.; Chernov, S.V. Electrical Properties of Solid Solutions in the PbF2–CdF2 System. Izv. Akad. Nauk, Neorg. Mater. 1982, 18, 168–169. (In Russian) [Google Scholar]
- Sorokin, N.I.; Buchinskaya, I.I.; Sobolev, B.P. The Ionic Conductivity of the Single Crystals of Pb0.67Cd0.33F2 and Pb0.67Cd0.33F2: Ce3+. Russ. J. Inorg. Chem. 1992, 37, 1368–1370. [Google Scholar]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic Computing System JANA2006: General Features. Z. Kristallogr. Cryst. Mater. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Barsoukov, E.; Macdonald, J.R. Impedance Spectroscopy: Theory, Experiment, and Applications; John Wiley & Sons: New York, NY, USA, 2005; 608p, Available online: https://books.google.ru/books?id=8hNkOWO_DLwC (accessed on 5 May 2023).
- Krukowska-Fulde, B.; Niemyski, T. Preparation and some properties of CdF2 single crystals. J. Cryst. Growth 1967, 1, 183–186. [Google Scholar] [CrossRef]
- Kaiser, W.; Spitzer, W.G.; Kaiser, R.H.; Howarth, L.E. Infrared Properties of CaF2, SrF2, and BaF2. Phys. Rev. 1962, 127, 1950–1954. [Google Scholar] [CrossRef]
- Chalmers, B. Principles of Solidification; Wiley: New York, NY, USA, 1964; 319р. [Google Scholar] [CrossRef]
- Fedorov, P.P. Morphological Stability of the Solidification Front near Minima and Maxima in the Liquidus Line in Binary Solid-Solution Systems. Inorg. Mater. 2001, 37, 84–92. [Google Scholar] [CrossRef]
- Kuznetsov, S.V.; Fedorov, P.P. Morphological stability of solid-liquid interface during melt crystallization of M1−xRxF2+x solid solutions. Inorg. Mater. 2008, 44, 1434–1458. [Google Scholar] [CrossRef]
- Fedorov, P.P.; Ushakov, S.N.; Uslamina, M.A.; Chernova, E.V.; Kuznetsov, S.V.; Voronov, V.V.; Düvel, A.; Heitjans, P.; Pynenkov, A.A.; Nishchev, K.N.; et al. Morphological Stability of the Solid–Liquid Interface during Melt Crystallization of Ca1–xSrxF2 Solid Solution. Crystallogr. Rep. 2018, 63, 837–843. [Google Scholar] [CrossRef]
- Ren, G.; Shen, D.; Wang, S.; Yin, Z. Structural defects and characteristics of lead fluoride (PbF2) crystals grown by non-vacuum Bridgman method. J. Cryst. Growth 2002, 243, 539–545. [Google Scholar] [CrossRef]
- Ren, G.; Shen, D.; Wang, S.; Yin, Z. Optical absorption on cubic 𝛽-PbF2 crystals. Chinese Phys. Lett. 2001, 18, 976–978. [Google Scholar] [CrossRef]
- Ren, G.; Qun, D.; Li, Z.; Shen, D. Transmission loss of lead fluoride crystals induced by oxygen contamination. J. Cryst. Growth 2003, 247, 141–147. [Google Scholar] [CrossRef]
- Dygas, J.R.; Breiter, M.W. Measurements of Large Impedances in a Wide Temperature and Frequency Range. Electrochim. Acta 1996, 41, 993–1001. [Google Scholar] [CrossRef]
- Matsulev, A.I.; Ivanov, Y.N.; Livshits, A.I.; Buznik, V.M.; Fedorov, P.P.; Buchinskaya, I.I.; Sobolev, B.P. Structural features of a crystalline solid solution Pb0.67Cd0.33F2 according to 19F NMR data. Russ. J. Inorg. Chem. 2000, 45, 296–298. (In Russian) [Google Scholar]
- Picinin, A.; Silva, M.A.P.; Rino, J.P. The structural inhomogeneity of lead–cadmium fluoride systems and its implications. J. Phys. Condens. Matter. 2009, 21, 335107. [Google Scholar] [CrossRef] [PubMed]
- Petrov, A.V.; Salamatov, M.S.; Murin, I.V.; Ivanov-Shitz, A.K. Nanoscale effects in PbF2–CdF2 Solid Solutions. Crystallogr. Rep. 2019, 64, 932–936. [Google Scholar] [CrossRef]
- Gotlib, I.Y.; Murin, I.V.; Piotrovskaya, E.M.; Brodskaya, E.N. Molecular Dynamics Simulations of Ba1-xGdxF2+x Solid Solutions over a Wide Temperature Range: Thermodynamic and Transport Properties. Inorg. Mater. 2001, 27, 975–978. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- RU 2 436 122 C1. Method of Producing Scintillation Ceramic and Scintillator. Available online: https://patents.s3.yandex.net/RU2436122C1_20111210.pdf (accessed on 10 August 2023).







| The Compositions of Crystals Grown in the MF2–M′F2 and MF2–M′F2–M″F2 Systems | Melting Point, K | Literature Sources |
|---|---|---|
| Ca0.582Sr0.418F2 | 1646 | [1,5] |
| Ca0.59Sr0.41F2 | - | [6,7] |
| Ca0.5Sr0.5F2 | - | [8] |
| Ca1−xSrxF2 (0 ≤ x ≤ 1) Ca0.257Sr0.743F2—studied composition | - | [9,10] |
| Ba0.66Sr0.34F2 | 1850 | [11] |
| Cd0.75Sr0.25F2 | 1298 | [12] |
| Cd0.77Sr0.23F2 | - | [7,13] |
| Pb0.67Cd0.33F2 | 1023 ± 5 | [7] |
| Ca0.3(3)Sr0.3(3)Ba0.3(3)F2 | 1423–1483 | [14,15] |
| No | The Crystal Composition, mol. % | a, Å | ρcalc., g/cm3 | n (λ = 632.8 nm) | |||||
|---|---|---|---|---|---|---|---|---|---|
| By Charge | According to XRFA (Center) | ||||||||
| PbF2 | CdF2 | SrF2 | PbF2 | CdF2 | SrF2 | ||||
| 1 | 63 | 31 | 6 | 62.1 | 30 | 7.9 | 5.7552(2) | 7.2212(5) | 1.7084 |
| 2 | 60 | 30 | 10 | 58.9 | 29.2 | 11.9 | 5.7606(3) | 7.0611(5) | 1.6862 |
| 3 | 58.5 | 29.3 | 12.2 | 59.1 | 27.7 | 13.2 | 5.7678(3) | 7.0301(5) | 1.6963 |
| 4 | 55 | 27 | 18 | 55.2 | 27.3 | 17.5 | 5.7717(8) | 6.8514(5) | 1.6792 |
| 5 | 53.6 | 39.8 | 6.6 | 51.6 | 39.8 | 8.5 | 5.7024(3) | 7.0566(5) | 1.6823 |
| 6 | 33.5 | 55 | 11.5 | 33 | 54.1 | 12.9 | 5.6406(2) | 6.6039(5) | 1.6403 |
| 7 | 66.7 | 33.3 | 0 | 66.7 | 33.3 | 0 | 5.7317(3) | 7.5232(5) | 1.7049 |
| 8 | 100 | 0 | 0 | 100 | 0 | 0 | 5.939(4) | 7.7706(5) | 1.7611 |
| 9 | 0 | 100 | 0 | 0 | 100 | 0 | 5.388(2) | 6.3849(5) | 1.5726 [35] |
| 10 | 0 | 0 | 100 | 0 | 0 | 100 | 5.800(2) | 4.2750(5) | 1.4371 [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buchinskaya, I.I.; Goryachuk, I.O.; Sorokin, N.I.; Sokolov, V.I.; Karimov, D.N. PbF2–CdF2–SrF2 Ternary Solid Solution: Crystal Growth and Investigation. Condens. Matter 2023, 8, 73. https://doi.org/10.3390/condmat8030073
Buchinskaya II, Goryachuk IO, Sorokin NI, Sokolov VI, Karimov DN. PbF2–CdF2–SrF2 Ternary Solid Solution: Crystal Growth and Investigation. Condensed Matter. 2023; 8(3):73. https://doi.org/10.3390/condmat8030073
Chicago/Turabian StyleBuchinskaya, Irina I., Ivan O. Goryachuk, Nikolay I. Sorokin, Victor I. Sokolov, and Denis N. Karimov. 2023. "PbF2–CdF2–SrF2 Ternary Solid Solution: Crystal Growth and Investigation" Condensed Matter 8, no. 3: 73. https://doi.org/10.3390/condmat8030073