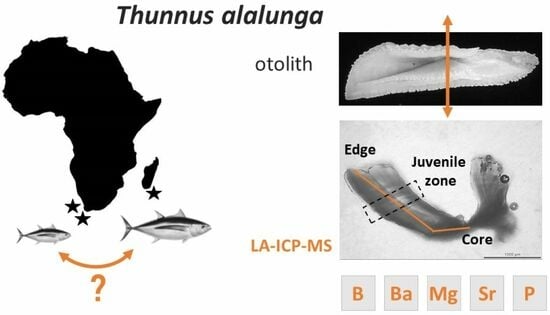
New Insights in Lifetime Migrations of Albacore Tuna (Thunnus alalunga, Bonnaterre, 1788) between the Southwest Indian and the Southeast Atlantic Oceans Using Otolith Microchemistry
Abstract
:1. Introduction
2. Material and Methods
2.1. Sampling
2.2. Otolith Preparation
2.3. Trace Element Analyses
2.4. Statistical Analyses
3. Results
3.1. Capture Location Signatures
3.2. Fish Spawning Origin
3.3. Fish Nursery Origin
3.4. Lifetime Migrations
4. Discussion
4.1. Reliability of the Elemental f Signatures of the Capture Zones
4.2. Potential Spawning Sites
4.3. Potential Nursery Sites
4.4. Connectivity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kerr, L.A.; Hintzen, N.T.; Cadrin, S.; Clausen, L.W.; Dickey-Collas, M.; Goethel, D.R.; Hatfield, E.M.; Kritzer, J.P.; Nash, R.D. Lessons learned from practical approaches to reconcile mismatches between biological population structure and stock units of marine fish. ICES J. Mar. Sci. 2017, 74, 1708–1722. [Google Scholar] [CrossRef]
- Rooker, J.R.; Arrizabalaga, H.; Fraile, I.; Secor, D.H.; Dettman, D.L.; Abid, N.; Addis, P.; Deguara, S.; Karakulak, F.; Kimoto, A.; et al. Crossing the line: Migratory and homing behaviors of Atlantic bluefin tuna. Mar. Ecol. Prog. Ser. 2014, 504, 265–276. [Google Scholar] [CrossRef]
- Rooker, J.R.; Dance, M.A.; Wells, R.J.; Ajemian, M.J.; Block, B.A.; Castleton, M.R.; Drymon, J.M.; Falterman, B.J.; Franks, J.S.; Hammerschlag, N.; et al. Population connectivity of pelagic megafauna in the Cuba-Mexico-United States triangle. Sci. Rep. 2019, 9, 1663. [Google Scholar] [CrossRef] [PubMed]
- ISSF. Status of the world fisheries for tuna: 22. In ISSF Technical Report 2022-13; International Seafood Sustainability Foundation: Washington, DC, USA, 20 July 2022. [Google Scholar]
- Stequert, B.; Marsac, F. Tropical Tuna Surface Fisheries in the Indian Ocean; FAO fisheries technical paper; FAO: Rome, Italy, 1989; Volume 282, 238p. [Google Scholar]
- Penney, A.; Yeh, S.; Kuo, C.; Leslie, R. Relationships between albacore (Thunnus alalunga) stocks in the southern Atlantic and Indian Oceans. Col. Vol. Sci. Pap. ICCAT 1998, 50, 261–271. [Google Scholar]
- Yeh, S.Y.; Hui, C.F.; Treng, T.D.; Kuo, C.L. Indian Ocean albacore stock structure studies by morphometric and DNA sequence methods. In Proceedings of the 6th Expert Consultation on Indian Ocean Tunas, Colombo, Sri Lanka, 25–29 September 1995; pp. 258–263. [Google Scholar]
- Nishikawa, Y.; Honma, M.; Ueyanagi, S.; Kikawa, S. Average distribution of larvae of oceanic species of scombroid fishes, 1956–1981. Bull. Far Seas Fish. Res. Lab. 1985, 12, 1–99. [Google Scholar]
- Nishida, T.; Tanaka, M. General reviews of Indian Ocean Albacore (Thunnus alalunga). IOTC-2008-WPTe-INF03. Available online: https://iotc.org/documents/general-reviews-indian-ocean-albacore-thunnus-alalunga-0 (accessed on 3 December 2023).
- Nikolic, N.; Montes, I.; Lalire, M.; Puech, A.; Bodin, N.; Arnaud-Haond, S.; Kerwath, S.; Corse, E.; Gaspar, P.; Hollanda, S.; et al. Connectivity and population structure of albacore tuna across southeast Atlantic and southwest Indian Oceans inferred from multidisciplinary methodology. Sci. Rep. 2020, 10, 15657. [Google Scholar] [CrossRef]
- IOTC. Improving Biological Knowledge of Albacore Tuna, Thunnus alalunga, in the Indian Ocean: A Scoping Study. IOTC-2022-WPTmT08(DP)-INF01. Available online: https://iotc.org/documents/WPTmT/801/INF01 (accessed on 3 December 2023).
- Dhurmeea, Z.; Zudaire, I.; Chassot, E.; Cedras, M.; Nikolic, N.; Bourjea, J.; West, W.; Appadoo, C.; Bodin, N. Reproductive Biology of Albacore Tuna (Thunnus alalunga) in the Western Indian Ocean. PLoS ONE 2016, 11, e0168605. [Google Scholar] [CrossRef]
- Chen, I.C.; Lee, P.F.; Tzeng, W. Distribution of albacore (Thunnus alalunga) in the Indian Ocean and its relation to environmental factors. Fish. Oceanogr. 2005, 14, 71–80. [Google Scholar] [CrossRef]
- Nikolic, N.; Fonteneau, A.; Hoarau, L.; Morandeau, G.; Puech, A.; Bourjea, J. Short Review on Biology, Structure, and Migration of Thunnus alalunga in the Indian Ocean. IOTC–2014–WPTmT05–13 Rev_2. Available online: http://www.iotc.org/fr/documents/short-review-biology-structure-and-migration-thunnus-alalunga-indian-ocean (accessed on 3 December 2023).
- Nikolic, N.; Morandeau, G.; Hoarau, L.; West, W.; Arrizabalaga, H.; Hoyle, S.; Nicol, S.J.; Bourjea, J.; Puech, A.; Farley, J.H.; et al. Review of albacore tuna, Thunnus alalunga, biology, fisheries and management. Rev. Fish. Sci. 2017, 27, 775–810. [Google Scholar] [CrossRef]
- Morita, S. On the relationship between the albacore stocks of the South Atlantic and Indian Oceans. Collect. Vol. Sci. Pap. ICCAT 1977, 7, 232–237. [Google Scholar]
- ICCAT. Report of the 2013 ICCAT north and south atlantic albacore stock assessment meeting. Collect. Vol. Sci. Pap. ICCAT 2014, 70, 830–995. [Google Scholar]
- ICCAT. Report for Biennial Period, 2020-21, Part II. ICCAT-SRS 2020; International Commission for the Conservation of Atlantic Tunas: Madrid, Spain, 2020; 956p. [Google Scholar]
- Ward, R.D. Genetics in fisheries management. Hydrobiologia 2000, 420, 191–201. [Google Scholar] [CrossRef]
- Montes, I.; Iriondo, M.; Manzano, C.; Arrizabalaga, H.; Jimenez, E.; Pardo, M.; Goñi, N.; Davies, C.; Estonba, A. Worldwide genetic structure of albacore Thunnus alalunga revealed by microsatellite DNA markers. Mar. Ecol. Prog. Ser. 2012, 471, 183–191. [Google Scholar] [CrossRef]
- Laconcha, U.; Iriondo, M.; Arrizabalaga, H.; Manzano, C.; Markaide, P.; Montes, I.; Zarraonaindia, I.; Velado, I.; Bilbao, E.; Goñi, N.; et al. New Nuclear SNP Markers Unravel the Genetic Structure and Effective Population Size of Albacore Tuna (Thunnus alalunga). PLoS ONE 2015, 10, e0128247. [Google Scholar] [CrossRef]
- Albaina, A.; Iriondo, M.; Velado, I.; Laconcha, U.; Zarraonaindia, I.; Arrizabalaga, H.; Pardo, M.A.; Lutcavage, M.; Grant, W.S.; Estonba, A. Single nucleotide polymorphism discovery in albacore and Atlantic bluefin tuna provides insights into worldwide population structure. Anim. Genet. 2013, 44, 678–692. [Google Scholar] [CrossRef]
- Clément, C.; Guiraud, H.; Incabi, L.; Loze, L.; Simonet, L. Inference about genetic demographic and population management of albacore. Master Aquatic Ecosystem Dynamics; University of Pau and the Adour Region: Pau, France, 2022; 60p. [Google Scholar]
- Arrizabalaga, H.; Costas, E.; Juste, J.; González-Garcés, A.; Nieto, B.; López-Rodas, V. Population structure of albacore Thunnus alalunga inferred from blood groups and tag-recapture analyses. Mar. Ecol. Prog. Ser. 2004, 282, 245–252. [Google Scholar] [CrossRef]
- Sturrock, A.M.; Trueman, C.N.; Darnaude, A.M.; Hunter, E. Can otolith elemental chemistry retrospectively track migrations in fully marine fishes? J. Fish Biol. 2012, 81, 766–795. [Google Scholar] [CrossRef]
- Avigliano, E. Optimizing the methodological design in fish stock delineation from otolith chemistry: Review of spatio-temporal analysis scales. Rev. Fish. Sci. Aquac. 2022, 30, 330–345. [Google Scholar] [CrossRef]
- Hazen, E.L.; Maxwell, S.M.; Bailey, H.; Bograd, S.J.; Hamann, M.; Gaspar, P.; Godley, B.; Shillinger, G.L. Ontogeny in marine tagging and tracking science: Technologies and data gaps. Mar. Ecol. Prog. Ser. 2012, 457, 221–240. [Google Scholar] [CrossRef]
- Kalish, J.M. Otolith microchemistry: Validation of the effects of physiology, age and environment on otolith composition. J. Exp. Mar. Biol. Ecol. 1989, 132, 151–178. [Google Scholar] [CrossRef]
- Campana, S. Chemistry and composition of fish otoliths: Pathways, mechanisms and applications. Mar. Ecol. Prog. Ser. 1999, 188, 263–297. [Google Scholar] [CrossRef]
- Walther, B.D.; Limburg, K.E.; Jones, C.M.; Schaffler, J.J. Frontiers in otolith chemistry: Insights, advances and applications. J. Fish Biol. 2017, 90, 473–479. [Google Scholar] [CrossRef]
- Reis-Santos, P.; Gillanders, B.M.; Sturrock, A.M.; Izzo, C.; Oxman, D.S.; Lueders-Dumont, J.A.; Hüssy, K.; Tanner, S.E.; Rogers, T.; Doubleday, Z.A.; et al. Reading the biomineralized book of life: Expanding otolith biogeochemical research and applications for fisheries and ecosystem-based management. Rev. Fish Biol. Fish. 2022, 33, 411–449. [Google Scholar] [CrossRef]
- Elsdon, T.; Wells, B.K.; Campana, S.E.; Gillanders, B.M.; Jones, C.M.; Limburg, K.E.; Secor, D.H.; Thorrold, S.R.; Walther, B.D. Otolith chemistry to describe movements and life-history parameters of fishes: Hypotheses, assumptions, limitations and inferences. Oceanogr. Mar. Biol. Annu. Rev. 2008, 46, 297–330. [Google Scholar]
- Baumann, H.; Wells, R.J.D.; Rooker, J.R.; Zhang, S.; Baumann, Z.; Madigan, D.J.; Fisher, N.S. Combining otolith microstructure and trace elemental analyses to infer the arrival of juvenile Pacific bluefin tuna in the California current ecosystem. ICES J. Mar. Sci. 2015, 72, 2128–2138. [Google Scholar] [CrossRef]
- Fraile, I.; Arrizabalaga, H.; Santiago, J.; Goñi, N.; Arregi, I.; Madinabeitia, S.; Wells, R.J.D.; Rooker, J.R. Otolith chemistry as an indicator of movements of albacore (Thunnus alalunga) in the North Atlantic Ocean. Mar. Freshw. Res. 2016, 67, 1002–1013. [Google Scholar] [CrossRef]
- Rooker, J.R.; Wells, D.; Itano, D.G.; Thorrold, S.R.; Lee, J.M. Natal origin and population connectivity of bigeye and yellowfin tuna in the Pacific Ocean. Fish. Oceanogr. 2016, 25, 277–291. [Google Scholar] [CrossRef]
- Artetxe-Arrate, I.; Fraile, I.; Crook, D.A.; Zudaire, I.; Arrizabalaga, H.; Greig, A.; Murua, H. Otolith microchemistry: A useful tool for investigating stock structure of yellowfin tuna (Thunnus albacares) in the Indian Ocean. Mar. Freshw. Res. 2019, 70, 1708–1721. [Google Scholar] [CrossRef]
- Xu, Y.; Sippel, T.; Teo, S.L.; Piner, K.; Chen, K.S.; Wells, R.J. A comparison study of North Pacific albacore (Thunnus alalunga) age and growth among various sources. In Proceedings of the Chinese Taipei: ISC Albacore Working Group Meeting, La Jolla, CA, USA, 14–28 April 2014. [Google Scholar]
- Ueyanagi, S. Observations on the distribution of tuna larva in the Indo-Pacific Ocean with emphasis on the delineation of spawning areas of albacore, Thunnus alalunga. Bull. Far Seas Fish. Res. Lab. 1969, 2, 177–219. [Google Scholar]
- Wu, C.L.; Kuo, C.L. Maturity and fecundity of albacore, Thunnus alalunga (Bonnaterre), from the Indian Ocean. J. Fish. Soc. Taiwan 1993, 20, 135–151. [Google Scholar]
- Jochum, K.P.; Weis, U.; Stoll, B.; Kuzmin, D.; Yang, Q.; Raczek, I.; Jacob, D.E.; Stracke, A.; Birbaum, K.; Frick, D.A.; et al. Determination of Reference Values for NIST SRM 610–617 Glasses Following ISO Guidelines. Geostand. Anal. Res. 2011, 35, 397–429. [Google Scholar] [CrossRef]
- Sirot, C.; Ferraton, F.; Panfil, I.J.; Childs, A.R.; Guilhaumon, F.; Darnaude, A.M. ElementR: An R package for reducing elemental data from LA-ICPMS analysis of biological calcified structures. Methods Ecol. Evol. 2017, 8, 1659–1667. [Google Scholar] [CrossRef]
- Fink-Jensen, P.; Jansen, T.; Thomsen, T.B.; Serre, S.H.; Hüssy, K. Marine chemistry variation along Greenland’s coastline indicated by chemical fingerprints in capelin (Mallotus villosus) otoliths. Fish. Res. 2021, 236, 105839. [Google Scholar] [CrossRef]
- Ramírez-Álvarez, R.; Contreras, S.; Vivancos, A.; Reid, M.; López-Rodríguez, R.; Górski, K. Unpacking the complexity of longitudinal movement and recruitment patterns of facultative amphidromous fish. Sci. Rep. 2022, 12, 3164. [Google Scholar] [CrossRef]
- Brophy, D.; Jeffries, T.E.; Danilowicz, B.S. Elevated manganese concentrations at the cores of clupeid otoliths: Possible environmental, physiological, or structural origins. Mar. Biol. 2004, 144, 779–786. [Google Scholar] [CrossRef]
- Ruttenberg, B.I.; Hamilton, S.L.; Hickford, M.J.; Paradis, G.L.; Sheehy, M.S.; Standish, J.D.; Ben-Tzvi, O.; Warner, R.R. Elevated levels of trace elements in cores of otoliths and their potential for use as natural tags. Mar. Ecol. Prog. Ser. 2005, 297, 273–281. [Google Scholar] [CrossRef]
- Macdonald, J.I.; Shelley, J.M.G.; Crook, D.A. A method for improving the estimation of natal chemical signatures in otoliths. Trans. Am. Fish. Soc. 2008, 137, 1674–1682. [Google Scholar] [CrossRef]
- Kaji, T.; Tanaka, M.; Oka, M.; Takeuchi, H.; Ohsumi, S.; Teruya, K.; Hirokawa, H. Growth and Morphological Development of Laboratory-Reared Yellowfin Tuna Thunnus albacares Larvae and Early Juveniles, with Special Emphasis on the Digestive System. Fish. Sci. 1999, 65, 700–707. [Google Scholar] [CrossRef]
- Itoh, T.; Shiina, Y.; Tsuji, S.; Endo, F.; Tezuka, N. Otolith daily increment formation in laboratory reared larval and juvenile bluefin tuna Thunnus thynnus. Fish. Sci. 2000, 66, 834–839. [Google Scholar] [CrossRef]
- García, A.; Cortés, D.; Ramírez, T.; Fehri-Bedoui, R.; Alemany, F.; Rodríguez, J.M.; Carpena, Á.; Álvarez, J.P. First data on growth and nucleic acid and protein content of field-captured Mediterranean bluefin (Thunnus thynnus) and albacore (Thunnus alalunga) tuna larvae: A comparative study. Sci. Mar. 2006, 70, 67–78. [Google Scholar] [CrossRef]
- Longhurst, A. Ecological Geography of the Sea; Academic Press: London, UK, 2007. [Google Scholar]
- Reygondeau, G.; Longhurst, A.; Martinez, E.; Beaugrand, G.; Antoine, D.; Maury, O. Dynamic biogeochemical provinces in the global ocean. Glob. Biogeochem. Cycles 2013, 17, 1046–1058. [Google Scholar] [CrossRef]
- Sturrock, A.M.; Hunter, E.; Milton, J.A.; Johnson, R.; Waring, C.P.; Trueman, C.N. Quantifying physiological influences on otolith microchemistry. Methods Ecol. Evol. 2015, 6, 806–816. [Google Scholar] [CrossRef]
- Reis-Santos, P.; Vasconcelos, R.P.; Tanner, S.E.; Fonseca, V.F.; Cabral, H.N.; Gillanders, B.M. Extrinsic and intrinsic factors shape the ability of using otolith chemistry to characterize estuarine environmental histories. Mar. Environ. Res. 2018, 140, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Rooker, J.R.; Secor, D.H. Otolith Microchemistry. In The Future of Bluefin Tunas: Ecology, Fisheries Management, and Conservation; Block, B.A., Ed.; JHU Press: Baltimore, MD, USA, 2019; Volume 45. [Google Scholar]
- Beardsley, J.G.L. Proposed migrations of albacore, Thunnus alalunga, in the Atlantic Ocean. Trans. Am. Fish. Soc. 1969, 98, 589–598. [Google Scholar] [CrossRef]
- Bard, F.X. L’habitat du germon (Thunnus alalunga) en Océan Atlantique. Col. Vol. Sci. Pap. ICCAT 1982, 17, 487–490. [Google Scholar]
- Reglero, P.; Tittensor, D.P.; Álvarez-Berastegui, D.; Aparicio-González, A.; Worm, B. Worldwide distributions of tuna larvae: Revisiting hypotheses on environmental requirements for spawning habitats. Mar. Ecol. Prog. Ser. 2014, 501, 207–224. [Google Scholar] [CrossRef]
- Bard, F.X.; Stretta, J.M. Résumé des connaissances actuelles sur la biologie et la pêche des thons tropicaux en Atlantique. Arch. Sci. Cent. Rech. Océanographiques Abidj. 1981, 7, 1–37. [Google Scholar]
- Woodson, L.E.; Wells, B.K.; Grimes, C.B.; Franks, R.P.; Santora, J.A.; Carr, M.H. Water and otolith chemistry identify exposure of juvenile rockfish to upwelled waters in an open coastal system. Mar. Ecol. Prog. Ser. 2013, 473, 261–273. [Google Scholar] [CrossRef]
- Wheeler, S.; Russell, A.D.; Fehrenbacher, J.S.; Morgan, S.G. Evaluating chemical signatures in a coastal upwelling region to reconstruct water mass associations of settlement-stage rockfishes. Mar. Ecol. Prog. Ser. 2016, 550, 191–206. [Google Scholar] [CrossRef]
- Labonne, M.; Masski, H.; Talba, S.; Tai, I.; Manchih, K.; Chfiri, R.; Lae, R. Major population’s separation area for sardine (Sardina pilchardus) and hake (Merluccius merluccius) revealed using otolith geochemistry on the Atlantic coast of Morocco. Fish. Res. 2022, 254, 10641. [Google Scholar] [CrossRef]
- IOTC. Indian Ocean albacore stock: Review of its fishery, biological data and results of its 2014 stock assessment. In Proceedings of the Indian Ocean Tuna Conservation, Shanghai, China, 18–21 July 2016. IOTC–2016–WPTmT06–09. [Google Scholar]
- IOTC. Spatiotemporal Distribution of Albacore in Relation to Oceanographic Variables in the Indian Ocean. IOTC-2019-WPTmT07-INF03. Available online: https://iotc.org/meetings/7th-working-party-temperate-tuna-wptmt-data-preparatory-meeting (accessed on 3 December 2023).
- Kamykowski, D.; Zentara, S.J.; Morrison, J.M.; Switzer, A.C. Dynamic global patterns of nitrate, phosphate, silicate, and iron availability and phytoplankton community composition from remote sensing data. Glob. Biogeochem. Cycles 2002, 16, 1077. [Google Scholar] [CrossRef]
- Barrett, P.M.; Resing, J.A.; Grand, M.M.; Measures, C.I.; Landing, W.M. Trace element composition of suspended particulate matter along three meridional CLIVAR sections in the Indian and Southern Oceans: Impact of scavenging on Al distributions. Chem. Geol. 2018, 502, 15–28. [Google Scholar] [CrossRef]
- Le Gall, J.Y. Expose Synoptique des Données Biologiques sur le Germon Thunnus alalunga (Bonaterre, 1788) de l’Ocean Atlantique (No. 109); Organisation des Nations Unies pour l’Alimentation et l’Agriculture, FAO: Rome, Italy, 1974; Volume 109, 70p. [Google Scholar]
- Coimbra, M.R.M. Proposed mouvements of albacore, Thunnus alalunga in south atlantic Ocean. SCRS/1998/040 Col. Vol. Sci. Pap. ICCAT 1999, 49, 97–136. [Google Scholar]
- Travassos, P. Anomalies thermiques et peche du germon (Thunnus alalunga) dans l’Atlantique tropical sud est. SCRS/1998/107 Col. Vol. Sci. Pap. ICCAT 1999, 49, 324–338. [Google Scholar]
- Lebeau, A. Etude de la biologie du germon de l’océan indien. Sci. Pêche 1971, 204, 1–10. [Google Scholar]
- Alonso, C.; Arrizabalaga, H.; Restrepo, V.R. Contribution of a chapter on Albacore Tuna for the revised ICCAT field manual. Col. Vol. Sci. Pap. ICCAT 2005, 58, 1646–1661. [Google Scholar]









| Location | N | Sampling Dates | FL Range (cm) | * Estimated Age Range (years) |
|---|---|---|---|---|
| southwest Indian Ocean (SWI-Feb 18) | 13 | February 2018 | 96–104 | 7–10 |
| southwest Indian Ocean (SWI-May 18) | 13 | May 2018 | 98–113 | 7–15+ |
| southwest Indian Ocean (SWI-Dec 18) | 20 | December 2018 | 96–116 | 7–15+ |
| South Africa—Hout Bay (SA-S) | 13 | March–April 2018 | 63–85 | 2–5 |
| South Africa—Saldanha Bay (SA-N) | 13 | March–April 2018 | 63–85 | 2–5 |
| SA-N | SA-S | Total SA | SWI-Feb | SWI SWI-May | SWI-Dec | Total SWI | Total All Samples | |
|---|---|---|---|---|---|---|---|---|
| SpO-1 | 83% | 15% | 46% | 85% | 8% | - | 28% | 35% |
| SpO-2 | 17% | 85% | 54% | 15% | 92% | 100% | 72% | 65% |
| Total | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% |
| SA-N | SA-S | Total SA | SWI-Feb | SWI SWI-May | SWI-Dec | Total SWI | Total All Samples | |
|---|---|---|---|---|---|---|---|---|
| Nursery 1 | 77% | 23% | 50% | 38% | 8% | 10% | 17% | 30% |
| Nursery 2 | 15% | 38% | 27% | 62% | 70% | 40% | 54% | 44% |
| Nursery 3 | 8% | 39% | 23% | - | 22% | 50% | 29% | 26% |
| Total | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% |
| Spawning Origin | Nursery 1 | Nursery 2 | Nursery 3 |
|---|---|---|---|
| SpO-1 | 76% | 28% | - |
| SpO-2 | 24% | 72% | 100% |
| Total | 100% | 100% | 100% |
| Spawning | Nursery | Migration Strategy | Total | SWI | Total | Total | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Origin | SA-N | SA-S | SA | SWI-Feb | SWI-May | SWI-Dec | SWI | All Samples | ||
| N1 | 1-SpO1-N1 | 70% | 7% | 38% | 38% | 8% | - | 13% | 22% | |
| SpO-1 | N2 | 2-SpO1-N2 | 7% | 7% | 8% | 47% | 8% | - | 13% | 12% |
| N3 | 3-SpO1-N3 | - | - | - | - | - | - | - | - | |
| N1 | 4-SpO2-N1 | 7% | 15% | 11% | - | - | 10% | 3% | 7% | |
| SpO-2 | N2 | 5-SpO2-N2 | 8% | 31% | 19% | 15% | 62% | 40% | 43% | 32% |
| N3 | 6-SpO2-N3 | 8% | 38% | 23% | - | 22% | 50% | 28% | 26% | |
| Total | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labonne, M.; Darnaude, A.M.; Fily, T.; Petit, C.; Nikolic, N.; Parker, D.; Norman, S.J.; Clear, N.; Farley, J.; Eveson, J.P.; et al. New Insights in Lifetime Migrations of Albacore Tuna (Thunnus alalunga, Bonnaterre, 1788) between the Southwest Indian and the Southeast Atlantic Oceans Using Otolith Microchemistry. Fishes 2024, 9, 38. https://doi.org/10.3390/fishes9010038
Labonne M, Darnaude AM, Fily T, Petit C, Nikolic N, Parker D, Norman SJ, Clear N, Farley J, Eveson JP, et al. New Insights in Lifetime Migrations of Albacore Tuna (Thunnus alalunga, Bonnaterre, 1788) between the Southwest Indian and the Southeast Atlantic Oceans Using Otolith Microchemistry. Fishes. 2024; 9(1):38. https://doi.org/10.3390/fishes9010038
Chicago/Turabian StyleLabonne, Maylis, Audrey M. Darnaude, Theotime Fily, Cécile Petit, Natacha Nikolic, Denham Parker, Stewart James Norman, Naomi Clear, Jessica Farley, Jennifer Paige Eveson, and et al. 2024. "New Insights in Lifetime Migrations of Albacore Tuna (Thunnus alalunga, Bonnaterre, 1788) between the Southwest Indian and the Southeast Atlantic Oceans Using Otolith Microchemistry" Fishes 9, no. 1: 38. https://doi.org/10.3390/fishes9010038