Sex-Dependent Lipid Profile Differences in Skin Mucus between Non-Parental and Parental Discus Fish (Symphysodon haraldi) Determined by Lipidomics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Fish
2.2. Sample Collection
2.3. Chemicals and Quality Control Samples
2.4. Lipid Extraction Process
2.5. Lipid Data Processing
2.6. Bioinformatic and Statistical Analyses
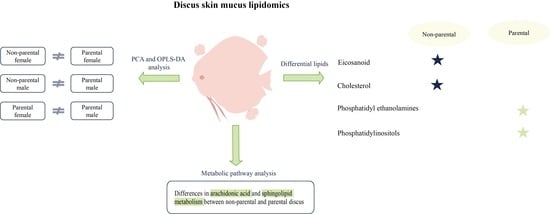
3. Results
3.1. Reliability of the Analytical Method
3.2. Analysis of the Lipid Profiles of Discus Fish Skin Mucus
3.3. PCA and OPLS-DA Analysis
3.4. Differential Lipids
3.5. Lipid Metabolism Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buckley, J. Biparental mucus feeding: A unique example of parental care in an Amazonian cichlid. J. Exp. Biol. 2010, 213, 3787–3795. [Google Scholar] [CrossRef] [PubMed]
- Schutz, M.; Barlow, G.W. Young of the Midas cichlid get biologically active nonnutrients by eating mucus from the surface of their parents. Fish Physiol. Biochem. 1997, 16, 11–18. [Google Scholar] [CrossRef]
- Chong, K.; Joshi, S.; Jin, L.T.; Shu-Chien, A.C. Proteomics profiling of epidermal mucus secretion of a cichlid (Symphysodon aequifasciata) demonstrating parental care behavior. Proteomics 2006, 6, 2251–2258. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Zhou, J.Q.; Gao, J.Z.; Chen, H.R.; Shen, Y.Q.; Chen, Z.Z. Sex-dependent changes in the skin mucus metabolome of discus fish (Symphysodon haraldi) during biparental care. J. Proteom. 2020, 221, 103784. [Google Scholar] [CrossRef] [PubMed]
- Brinchmann, M.F. Immune relevant molecules identified in the skin mucus of fish using -omics technologies. Mol. Biosyst. 2016, 12, 2056–2063. [Google Scholar] [CrossRef]
- Lewis, R.W. Fish cutaneous mucus: A new source of skin surface lipid. Lipids 1970, 5, 947–949. [Google Scholar] [CrossRef]
- Galabert, C.; Jacquot, J.; Zahm, J.M.; Puchelle, E. Relationships between the lipid content and the rheological properties of airway secretions in cystic fibrosis. Clin. Chim. Acta Int. J. Clin. Chem. 1987, 164, 139–149. [Google Scholar] [CrossRef]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef]
- Matsumura, K.M.S.; Fusetani, N. Possible involvement of phosphatidylcholine in school recognition in the catfish, Plotosus lineatus. Zool. Sci. 2004, 21, 257–264. [Google Scholar] [CrossRef]
- Tocher, D.R.; Bendiksen, E.A.; Campbell, P.J.; Bell, J.G. The role of phospholipids in nutrition and metabolism of teleost fish. Aquaculture 2008, 280, 21–34. [Google Scholar] [CrossRef]
- Geurden, I.; Marion, D.; Charlon, N.; Coutteau, P.; Bergot, P. Comparison of different soybean phospholipidic fractions as dietary supplements for common carp, Cyprinus carpio, larvae. Aquaculture 1998, 161, 225–235. [Google Scholar] [CrossRef]
- Field, F.J.; Mathur, S.N. Intestinal lipoprotein synthesis and secretion. Prog. Lipid Res. 1995, 34, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Yin, Y.D.; Xu, M.M.; Wang, R.H.; Zhu, Z.J. Absolute quantitative lipidomics reveals lipidome-wide alterations in aging brain. Metabolomics 2018, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Torrecillas, S.; Montero, D.; Dominguez, D.; Robaina, L.; Izquierdo, M. Skin Mucus Fatty Acid Composition of Gilthead Sea Bream (Sparus aurata): A Descriptive Study in Fish Fed Low and High Fish Meal Diets. Fishes 2019, 4, 15. [Google Scholar] [CrossRef]
- Sprague, M.; Desbois, A.P. Fatty acid and lipid class composition in cutaneous mucus of Atlantic salmon, Salmo salar (L.). Aquac. Res. 2021, 52, 6808–6813. [Google Scholar] [CrossRef]
- Sato, S.; Hirayama, T.; Hirazawa, N. Lipid content and fatty acid composition of the monogenean Neobenedenia girellae and comparison between the parasite and host fish species. Parasitology 2008, 135, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Chen, Z.; Qu, H.; Gao, J. Growth and fatty acid composition of discus fish Symphysodon haraldi given varying feed ratios of beef heart, duck heart, and shrimp meat. Aquac. Fish. 2018, 3, 84–89. [Google Scholar] [CrossRef]
- Ekman, D.R.; Skelton, D.M.; Davis, J.M.; Villeneuve, D.L.; Cavallin, J.E.; Schroeder, A.; Jensen, K.M.; Ankley, G.T.; Collette, T.W. Metabolite Profiling of Fish Skin Mucus: A Novel Approach for Minimally-Invasive Environmental Exposure Monitoring and Surveillance. Environ. Sci. Technol. 2015, 49, 3091–3100. [Google Scholar] [CrossRef]
- Ivanova, L.; Tartor, H.; Grove, S.; Kristoffersen, A.B.; Uhlig, S. Workflow for the Targeted and Untargeted Detection of Small Metabolites in Fish Skin Mucus. Fishes 2018, 3, 21. [Google Scholar] [CrossRef]
- Giaquinto, P.C.; Hara, T.J. Discrimination of bile acids by the rainbow trout olfactory system: Evidence as potential pheromone. Biol. Res. 2008, 41, 33–42. [Google Scholar] [CrossRef]
- Hara, T.J. The diversity of chemical stimulation in fish olfaction and gustation. Rev. Fish Biol. Fish. 1994, 4, 1–35. [Google Scholar] [CrossRef]
- Zhang, C.; Brown, S.B.; Hara, T.J. Biochemical and physiological evidence that bile acids produced and released by lake char (Salvelinus namaycush) function as chemical signals. J. Comp. Physiol. B 2001, 171, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.J.; Zhang, X.L.; Yan, X.J. Intestinal Bile Acids Induce Behavioral and Olfactory Electrophysiological Responses in Large Yellow Croaker (Larimichthys crocea). Fishes 2023, 8, 26. [Google Scholar] [CrossRef]
- Kasumyan, A.O.; Vinogradskaya, M.I. Palatability of Bile Substances for Fish. J. Ichthyol. 2019, 59, 610–618. [Google Scholar] [CrossRef]
- Gao, Y.J.; Yao, Y.F.; Huang, J.; Sun, Y.J.; Wu, Q.J.; Guo, D.Q.; Wang, S.P. Effect of dietary bile acids supplementation on growth performance, feed utilization, intestinal digestive enzyme activity and fatty acid transporters gene expression in juvenile leopard coral grouper (Plectropomus leopardus). Front. Mar. Sci. 2023, 10, 1171344. [Google Scholar] [CrossRef]
- Li, T.; Yan, X.B.; Dong, X.H.; Pan, S.M.; Tan, B.P.; Zhang, S.; Suo, X.X.; Li, Z.H.; Huang, W.B.; Yang, Y.Z.; et al. Choline Alleviates Disorders of Lipid Metabolism in Hybrid Grouper (female Epinephelus fuscoguttatus × male E. lanceolatus) Caused by High-Lipid Diet. Aquac. Nutr. 2022, 2022, 8998849. [Google Scholar] [CrossRef]
- Adam, A.H.; Verdegem, M.; Soliman, A.A.; Zaki, M.; Khalil, R.H.; Nour, A.M.; Khaled, A.A.; Basuini MF, E.; Khalil, H.S. Effect of dietary bile acids: Growth performance, immune response, genes expression of fatty acid metabolism, intestinal, and liver morphology of striped catfish (Pangasianodon hypophthalmus). Aquac. Rep. 2023, 29, 101510. [Google Scholar] [CrossRef]
- Buczynski, M.W.; Dumlao, D.S.; Dennis, E.A. An integrated omics analysis of eicosanoid biology. J. Lipid Res. 2009, 50, 1015–1038. [Google Scholar] [CrossRef]
- Funk, C.D. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 2001, 294, 1871–1875. [Google Scholar] [CrossRef]
- Han, X.; Chen, X.Y.; Sun, Y.J.; Nadler, J.L.; Bleich, D. Induction of cyclooxygenase-2 gene in Pancreatic β-cells by 12-lipoxygenase pathway product 12-hydroxyeicosatetraenoic acid. Mol. Endocrinol. 2002, 16, 2145–2154. [Google Scholar] [CrossRef]
- Wu, J.; Ding, D.H.; Li, Q.Q.; Wang, X.Y.; Sun, Y.Y.; Li, L.J. Lipoxin A4 Regulates Lipopolysaccharide-Induced BV2 Microglial Activation and Differentiation via the Notch Signaling Pathway. Front. Cell. Neurosci. 2019, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Das, U. Arachidonic Acid Has Anti-Inflammatory and Anti-Diabetic Actions In Vitro and In Vivo. Curr. Dev. Nutr. 2020, 4, 747. [Google Scholar] [CrossRef]
- Kronke, G.; Katzenbeisser, J.; Uderhardt, S.; Zaiss, M.M.; Scholtysek, C.; Schabbauer, G.; Zarbock, A.; Koenders, M.I.; Axmann, R.; Zwerina, J.; et al. 12/15-Lipoxygenase Counteracts Inflammation and Tissue Damage in Arthritis. J. Immunol. 2009, 183, 3383–3389. [Google Scholar] [CrossRef]
- Coutteau, P.; Geurden, I.; Camara, M.R.; Bergot, P.; Sorgeloos, P. Review on the dietary effects of phospholipids in fish and crustacean larviculture. Aquaculture 1997, 155, 149–164. [Google Scholar] [CrossRef]
- Sandel, E.; Nixon, O.; Lutzky, S.; Ginsbourg, B.; Tandler, A.; Uni, Z.; Koven, W. The effect of dietary phosphatidylcholine/phosphatidylinositol ratio on malformation in larvae and juvenile gilthead sea bream (Sparus aurata). Aquaculture 2010, 304, 42–48. [Google Scholar] [CrossRef]
- Vance, J.E. Thematic review series: Glycerolipids. Phosphatidylserine and phosphatidylethanolamine in mammalian cells: Two metabolically related aminophospholipids. J. Lipid Res. 2008, 49, 1377–1387. [Google Scholar] [CrossRef]
- Modica-Napolitano, J.S.; Renshaw, P.F. Ethanolamine and phosphoethanolamine inhibit mitochondrial function in vitro: Implications for mitochondrial dysfunction hypothesis in depression and bipolar disorder. Biol. Psychiatry 2004, 55, 273–277. [Google Scholar] [CrossRef]
- Torres, M.; Navarro, J.C.; Varo, I.; Agulleiro, M.J.; Morais, S.; Monroig, O.; Hontoria, F. Expression of genes related to long-chain (C18-22) and very long-chain (>C24) fatty acid biosynthesis in gilthead seabream (Sparus aurata) and Senegalese sole (Solea senegalensis) larvae: Investigating early ontogeny and nutritional regulation. Aquaculture 2020, 520. [Google Scholar] [CrossRef]
- Villalta, M.; Estevez, A.; Bransden, M.P.; Bell, J.G. Effects of dietary eicosapentaenoic acid on growth, survival, pigmentation and fatty acid composition in Senegal sole (Solea senegalensis) larvae during the Artemia feeding period. Aquac. Nutr. 2008, 14, 232–241. [Google Scholar] [CrossRef]
- Vizcaíno-Ochoa, V.; Lazo, J.P.; Barón-Sevilla, B.; Drawbridge, M.A. The effect of dietary docosahexaenoic acid (DHA) on growth, survival and pigmentation of California halibut Paralichthys californicus larvae (Ayres, 1810). Aquaculture 2010, 302, 228–234. [Google Scholar] [CrossRef]
- Liakh, I.; Pakiet, A.; Sledzinski, T.; Mika, A. Modern Methods of Sample Preparation for the Analysis of Oxylipins in Biological Samples. Molecules 2019, 24, 1639. [Google Scholar] [CrossRef] [PubMed]
- Grosvenor, C.E.; Picciano, M.F.; Baumrucker, C.R. Hormones and growth factors in milk. Endocr Rev 1993, 14, 710–728. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Xin, G.; Niannian, W.; Shiling, L.; Juan, D.; Zeliang, Q.; Junrong, Z.; Qingling, W. Evaluation of changes in egg yolk lipids during storage based on lipidomics through UPLC-MS/MS. Food Chem. 2023, 398, 133931. [Google Scholar]
- Tamura, Y.; Onguka, O.; Itoh, K.; Endo, T.; Iijima, M.; Claypool, S.M.; Sesaki, H. Phosphatidylethanolamine Biosynthesis in Mitochondria: Phosphatidylserine (PS) Trafficking is Independent of a PS Decarboxylase and Intermembrane Space Proteins Ups1p and Ups2p. J. Biol. Chem. 2012, 287, 43961–43971. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.; Wen, B.; Liang, H.; Gao, J.; Chen, Z. Sex-Dependent Lipid Profile Differences in Skin Mucus between Non-Parental and Parental Discus Fish (Symphysodon haraldi) Determined by Lipidomics. Fishes 2024, 9, 27. https://doi.org/10.3390/fishes9010027
Zhao S, Wen B, Liang H, Gao J, Chen Z. Sex-Dependent Lipid Profile Differences in Skin Mucus between Non-Parental and Parental Discus Fish (Symphysodon haraldi) Determined by Lipidomics. Fishes. 2024; 9(1):27. https://doi.org/10.3390/fishes9010027
Chicago/Turabian StyleZhao, Shichen, Bin Wen, Hua Liang, Jianzhong Gao, and Zaizhong Chen. 2024. "Sex-Dependent Lipid Profile Differences in Skin Mucus between Non-Parental and Parental Discus Fish (Symphysodon haraldi) Determined by Lipidomics" Fishes 9, no. 1: 27. https://doi.org/10.3390/fishes9010027