Investigating the Genetic and Dietary Factors Influencing Foot Muscle Color and Growth in Haliotis gigantea
Abstract
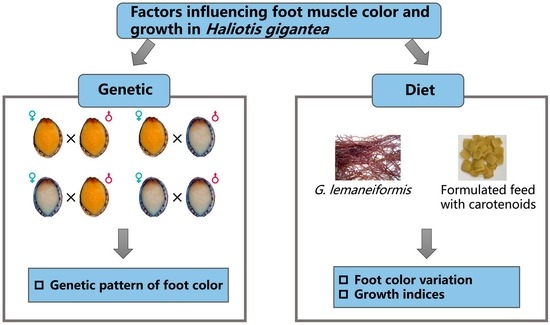
:1. Introduction
2. Materials and Methods
2.1. Parental Abalone Breeding and Offspring Cultivation
2.2. Color Identification of Abalone Foot Muscle
2.3. Experimental Design of Acclimation and Feeding of the Experimental Abalone
2.4. Assessing the Growth Indices of Abalone
- (1)
- Fatness (g/mm) = total weight (g)/shell length (mm)
- (2)
- Shell length gain ratio (%) = (final shell length (g) − initial shell length (g))/initial shell length (mm)
- (3)
- Total mass gain ratio (%) = (final total weight (g) − initial total weight (g))/initial total weight (g)
- (4)
- Fatness gain ratio (%) = (final fatness (g/mm) − initial fatness (g/mm))/initial fatness (g/mm)
2.5. Determination of Carotenoid Content in Abalone Foot Muscle
2.5.1. Isolation of Pigments
2.5.2. HPLC Analysis
2.6. Data Analysis
3. Results
3.1. Genetic Foot-Muscle Color Patterns
3.2. Effects of Feeds on the Foot Muscle Color
3.3. Effects of Feeds on the Growth Rate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, N.; Hu, J.; Wang, S.; Cheng, J.; Hu, X.; Lu, Z.; Lin, Z.; Zhu, W.; Bao, Z. Isolation and Identification of the Main Carotenoid Pigment from the Rare Orange Muscle of the Yesso Scallop. Food Chem. 2010, 118, 616–619. [Google Scholar] [CrossRef]
- Wei, X.; Zeng, W.; Tang, B.; He, J.; Chen, N.; Luo, X.; Feng, D.; You, W.; Ke, C. Comparative Analysis of the Predominant Carotenoids and Chemical Components in the Common and Orange-muscle Mutant of Haliotis gigantea. Aquac. Res. 2019, 50, 2938–2950. [Google Scholar] [CrossRef]
- Kantha, S.S. Carotenoids of Edible Molluscs: A review. J. Food Biochem. 1989, 13, 429–442. [Google Scholar] [CrossRef]
- Suzanne, T.W. Molluscan Shell Colour. Biol. Rev. 2017, 92, 1039–1058. [Google Scholar]
- Goodwin, T.W. The Biochemistry of the Carotenoids; Chapman and Hall: London, UK, 1984. [Google Scholar]
- Miki, W.; Yamaguchi, K.; Konosu, S.; Takane, T.; Satake, M.; Fujita, T.; Kuwabara, H.; Shimeno, S.; Takeda, M. Origin of Tunaxanthin in the Integument of Yellowtail (Seriola quinqueradiata). Comp. Biochem. Physiol. Part B Comp. Biochem. 1985, 80, 195–201. [Google Scholar] [CrossRef]
- Gouveia, L.; Rema, P. Effect of Microalgal Biomass Concentration and Temperature on Ornamental Goldfish (Carassius auratus) Skin Pigmentation. Aquac. Nutr. 2005, 11, 19–23. [Google Scholar] [CrossRef]
- Schubert, N.; Garcia-Mendoza, E.; Pacheco-Ruiz, I. Carotenoid Composition of Marine Red Algae. J. Phycol. 2006, 42, 1208–1216. [Google Scholar] [CrossRef]
- Sachindra, N.M.; Sato, E.; Maeda, H.; Hosokawa, M.; Niwano, Y.; Kohno, M.; Miyashita, K. Radical Scavenging and Singlet Oxygen Quenching Activity of Marine Carotenoid Fucoxanthin and Its Metabolites. J. Agric. Food Chem. 2007, 55, 8516–8522. [Google Scholar] [CrossRef]
- Chojnacka, K.; Saeid, A.; Witkowska, Z.; Tuhy, L. Biologically Active Compounds in Seaweed Extracts—The Prospects for the Application. Open. Conf. Proc. J. 2012, 3, 20–28. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Landrum, J.T.; Bone, R.A. Biologic Mechanisms of the Protective Role of Lutein and Zeaxanthin in the Eye. Annu. Rev. Nutr. 2003, 23, 171–201. [Google Scholar] [CrossRef]
- Carvalho, C.C.C.R.; de Caramujo, M.J. Carotenoids in Aquatic Ecosystems and Aquaculture: A Colorful Business with Implications for Human Health. Front. Mar. Sci. 2017, 4, 93. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, H.; Zhang, T.; Wang, S.; Sun, Z.; Liu, W.; Li, Y. Total Carotenoid Differences in Scallop Tissues of Chlamys nobilis (Bivalve: Pectinidae) with Regard to Gender and Shell Colour. Food Chem. 2010, 122, 1164–1167. [Google Scholar] [CrossRef]
- Yamada, S.; Tanaka, Y.; Sameshima, M.; Ito, Y. Pigmentation of Prawn (Penaeus japonicus) with Carotenoids. I. Effect of Dietary Astaxanthin, β-Carotene and Cantaxanthin on Pigmentation. Aquaculture 1990, 87, 323–330. [Google Scholar] [CrossRef]
- Yanar, Y.; Celik, M.; Yanar, M. Seasonal Changes in Total Carotenoid Contents of Wild Marine Shrimps (Penaeus semisulcatus and Metapenaeus monoceros) Inhabiting the Eastern Mediterranean. Food Chem. 2004, 88, 267–269. [Google Scholar] [CrossRef]
- Nègre-Sadargues, G.; Castillo, R.; Segonzac, M. Carotenoid Pigments and Trophic Behaviour of Deep-Sea Shrimps (Crustacean, Decapoda, Alvinocarididae) from a Hydrothermal Area of the Mid-Atlantic Ridge. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2000, 127A, 293–300. [Google Scholar] [CrossRef]
- Borisovets, E.E.; Zadorozhny, P.A.; Kalinina, M.V.; Lepskaya, N.V.; Yakush, E.V. Changes of Major Carotenoids in Gonads of Sea Urchins (Strongylocentrotus intermedius and S. nudus) at Maturation. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2002, 132, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Hendry, G.A.F.; Houghton, J.D. Natural Food Colorants; Chapman and Hall: London, UK, 1996. [Google Scholar]
- Bansemer, M.S.; Qin, J.G.; Harris, J.O.; Duong, D.N.; Hoang, T.H.; Howarth, G.S.; Stone, D.A.J. Growth and Feed Utilisation of Greenlip Abalone (Haliotis laevigata) Fed Nutrient Enriched Macroalgae. Aquaculture 2016, 452, 62–68. [Google Scholar] [CrossRef]
- Daume, S. The Roles of Bacteria and Micro and Macro Algae in Abalone Aquaculture: A Review. J. Shellfish Res. 2006, 25, 151–157. [Google Scholar] [CrossRef]
- Park, C.-J.; Kim, S.Y. Abalone Aquaculture in Korea. J. Shellfish Res. 2013, 32, 17–19. [Google Scholar] [CrossRef]
- Cuesta-Gomez, D.M.; Sánchez-Saavedra, M.D.P. Effects of Dietary Protein and Carbohydrate Levels on Gonad Index, Composition, and Color in the Purple Sea Urchin Strongylocentrotus purpuratus. N. Am. J. Aquac. 2018, 80, 193–205. [Google Scholar] [CrossRef]
- Kause, A.; Ritola, O.; Paananen, T.; Eskelinen, U.; Mäntysaari, E. Big and Beautiful? Quantitative Genetic Parameters for Appearance of Large Rainbow Trout. J. Fish Biol. 2003, 62, 610–622. [Google Scholar] [CrossRef]
- Katassonov, V.J.; Dementyev, V.N.; Klimov, A.V. Genetics of Color in the Ornamental Carp: The Inheritance of Underlying Dark Coloration. Russ. J. Genet. 2001, 37, 1210–1211. [Google Scholar] [CrossRef]
- Zhang, J.; Pan, G. Body Form and Body Colour in Hybrids of Cyprinus Carpio. J. Fish. China 1983, 7, 301–312. [Google Scholar]
- Taniguchi, N.; Kijima, A.; Tamura, T.; Takegami, K.; Yamasaki, I. Color, Growth and Maturation in Ploidy-Manipulated Fancy Carp. Aquaculture 1986, 57, 321–328. [Google Scholar] [CrossRef]
- Chen, M.; Liu, B.; Ma, B.; Liu, G.; Cao, W.; Liu, X.; Yan, X.; Yang, B.; Wang, C. Selection of a Carotenoid-Rich Scallop Strain, QN Orange, from the Inter-Specific Hybrids between the Bay Scallop and the Peruvian Scallop. Aquaculture 2020, 528, 735513. [Google Scholar] [CrossRef]
- Li, X.; Wang, S.; Xun, X.; Zhang, M.; Wang, S.; Li, H.; Zhao, L.; Fu, Q.; Wang, H.; Li, T.; et al. A Carotenoid Oxygenase is Responsible for Muscle Coloration in Scallop. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2019, 1864, 966–975. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Y.; Liu, S.; Li, M.; Yao, R.; Niu, S.; Yuan, J.; Wang, H.; Hu, J.; Bao, Z.; et al. Transcriptome and Network Analyses Reveal Key Pathways and Genes Involved in Response to Carotenoid Deposition in Scallop Muscle. Front. Mar. Sci. 2023, 10, 1158325. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, J.; Zhu, S.; Liu, X.; Ma, B.; Wang, C.; Xu, J.; Yan, X. Pigment Profiling of ‘QN Orange’scallop and ‘Bohai Red’ Scallop Adductor Muscles by Liquid Chromatography Coupled with Triple Quadrupole Mass Spectrometry. Aquaculture 2019, 508, 178–185. [Google Scholar] [CrossRef]
- Hoang, T.H.; Qin, J.G.; Stone, D.A.J.; Harris, J.O.; Duong, D.N.; Bansemer, M.S. Colour Changes of Greenlip Abalone (Haliotis laevigata donovan) Fed Fresh Macroalgae and Dried Algal Supplement. Aquaculture 2016, 456, 16–23. [Google Scholar] [CrossRef]
- Dang, V.T.; Li, Y.; Speck, P.; Benkendorff, K. Effects of Micro and Macroalgal Diet Supplementations on Growth and Immunity of Greenlip Abalone, Haliotis laevigata. Aquaculture 2011, 320, 91–98. [Google Scholar] [CrossRef]
- Hoang, T.H.; Stone, D.A.J.; Duong, D.N.; Bansemer, M.S.; Harris, J.O.; Qin, J.G. Colour Change of Greenlip Abalone (Haliotis laevigata donovan) Fed Formulated Diets Containing Graded Levels of Dried Macroalgae Meal. Aquaculture 2017, 468, 278–285. [Google Scholar] [CrossRef]
- Hoang, T.H.; Stone, D.A.J.; Duong, D.N.; Harris, J.O.; Qin, J.G. Changes of Body Colour and Tissue Pigments in Greenlip Abalone (Haliotis laevigata donovan) Fed Macroalgal Diets at Different Temperatures. Aquac. Res. 2020, 51, 5175–5183. [Google Scholar] [CrossRef]
- Bansemer, M.S.; Qin, J.G.; Harris, J.O.; Howarth, G.S.; Stone, D.A.J. Nutritional Requirements and Use of Macroalgae as Ingredients in Abalone Feed. Rev. Aquac. 2016, 8, 121–135. [Google Scholar] [CrossRef]
- Garcia-Esquivel, Z.; Felbeck, H. Comparative Performance of Juvenile Red Abalone, Haliotis rufescens, Reared in Laboratory with Fresh Kelp and Balanced Diets. Aquac. Nutr. 2009, 15, 209–217. [Google Scholar] [CrossRef]
- Wei, X.; Yang, B.; Zeng, W.; Tang, B.; Huang, M.; Luo, X.; You, W.; Ke, C. Carotenoid Accumulation in Common and Orange-Muscle Mutant of Abalone, Haliotis gigantea, Fed with Different Macroalgae. Aquac. Nutr. 2022, 9287594. [Google Scholar] [CrossRef]



| Parental Combination (Female × Male) | ||||
|---|---|---|---|---|
| RR × RR | SS × SS | RR × SS | SS × RR | |
| Progeny separation (SS:RR) | RR-1: 0:94 (0:1) RR-2: 9:852 (0:1) RR-3: 0:230 (0:1) RR-4: 0:450 (0:1) | SS-1: 130:0 (1:0) SS-2: 43:15 (3:1) SS-3: 312:97 (3:1) | RS-1: 163:0 (1:0) RS-2: 540:0 (1:0) RS-3: 850:0 (1:0) RS-4: 551:0 (1:0) RS-5: 137:0 (1:0) RS-6: 106:0 (1:0) | SR-1: 140:0 (1:0) SR-2: 39:47 (1:1) |
| Diet | Carotenoid (mg/kg) | |
|---|---|---|
| Zeaxanthin | -Carotene | |
| Formulated feed | 130.33 ± 3.68 a | 149.33 ± 4.11 a |
| G. lemaneiformis | 126.29 ± 15.96 a | 143.95 ± 8.73 a |
| Diet | Ash (%) | Moisture (%) | Crude Protein (%) | Crude Lipid (%) | Crude Carbohydrate (%) |
|---|---|---|---|---|---|
| Formulated feed | 10.69 ± 0.57 a | 6.55 ± 0.04 a | 40.00 ± 0.50 a | 3.58 ± 0.23 a | 39.18 ± 0.97 a |
| G. lemaneiformis | 17.52 ± 0.01 b | 90.37 ± 0.41 b | 15.91 ± 5.39 b | 1.74 ± 0.11 b | 64.83 ± 1.89 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, Y.; Zeng, W.; Gao, X.; Cai, M.; You, W. Investigating the Genetic and Dietary Factors Influencing Foot Muscle Color and Growth in Haliotis gigantea. Fishes 2023, 8, 443. https://doi.org/10.3390/fishes8090443
Ke Y, Zeng W, Gao X, Cai M, You W. Investigating the Genetic and Dietary Factors Influencing Foot Muscle Color and Growth in Haliotis gigantea. Fishes. 2023; 8(9):443. https://doi.org/10.3390/fishes8090443
Chicago/Turabian StyleKe, Yizhou, Wencui Zeng, Xiaolong Gao, Mingyi Cai, and Weiwei You. 2023. "Investigating the Genetic and Dietary Factors Influencing Foot Muscle Color and Growth in Haliotis gigantea" Fishes 8, no. 9: 443. https://doi.org/10.3390/fishes8090443