Honeycomb-like MnO2/Biochar Catalyst Fabricated by High-Energy Electron Beam Irradiation for Degradation of Antibiotics in Swine Urine
Abstract
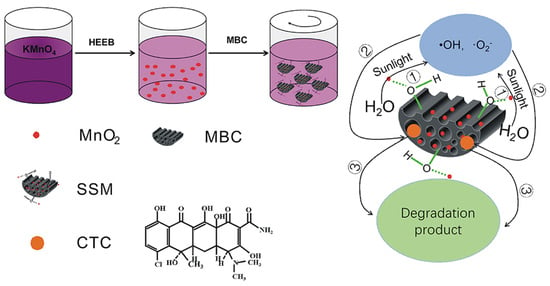
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of MBC, MnO2, MnO2/BC and MnO2/MBC
2.3. Degradation Performance Investigation
2.4. Reuse of SSM
2.5. Investigation of DE of SygSM on CTC in Swine Urine Aqueous Solution
2.6. Characterization
3. Results and Discussion
3.1. Morphological Observation of BC, MBC, and MnO2/MBC
3.2. Degradation of CTC by MnO2/MBC in Water
3.3. Interaction Analysis
3.4. Possible Degradation Mechanism Study
3.5. Degradation Performance of SSM on CTC in Swine Urine
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Tong, L.; Zhu, P.; Huang, P.; Tan, Z.; Qin, F.; Shi, W.; Wang, M.; Nie, H.; Yan, G.; et al. Adsorption of chlortetracycline onto biochar derived from corn cob and sugarcane bagasse. Water Sci. Technol. 2018, 78, 1336–1347. [Google Scholar] [CrossRef]
- Tanoue, R.; Sato, Y.; Motoyama, M.; Nakagawa, S.; Shinohara, R.; Nomiyama, K. Plant uptake of pharmaceutical chemicals detected in recycled organic manure and reclaimed wastewater. J. Agric. Food Chem. 2012, 60, 10203–10211. [Google Scholar] [CrossRef] [PubMed]
- Taheran, M.; Brar, S.K.; Verma, M.; Surampalli, R.Y.; Zhang, T.C.; Valero, J.R. Membrane processes for removal of pharmaceutically active compounds (PhACs) from water and wastewaters. Sci. Total Environ. 2016, 547, 60–77. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.L. Antibiotics and antibiotic resistance genes in natural environments. Science 2008, 321, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yao, L.; Ye, L.; Long, B.; Dai, Y.; Ding, Y. Benzimidazole-based hyper-cross-linked polymers for effective adsorption of chlortetracycline from aqueous solution. J. Environ. Chem. Eng. 2020, 8, 104562. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Xu, J.; Xie, Z.; Tang, J.; Li, X.; Fan, S. Simultaneous carbonization, activation, and magnetization for producing tea waste biochar and its application in tetracycline removal from the aquatic environment. J. Environ. Chem. Eng. 2021, 9, 105324. [Google Scholar] [CrossRef]
- Xu, Q.; Zhou, Q.; Pan, M.; Dai, L. Interaction between chlortetracycline and calcium-rich biochar: Enhanced removal by adsorption coupled with flocculation. Chem. Eng. J. 2020, 382, 122705. [Google Scholar] [CrossRef]
- You, N.; Yao, H.; Wang, Y.; Fan, H.T.; Wang, C.S.; Sun, T. Development and evaluation of diffusive gradients in thin films based on nano-sized zinc oxide particles for the in situ sampling of tetracyclines in pig breeding wastewater. Sci. Total Environ. 2019, 651, 1653–1660. [Google Scholar] [CrossRef]
- Zhu, X.D.; Wang, Y.J.; Sun, R.J.; Zhou, D.M. Photocatalytic degradation of tetracycline in aqueous solution by nanosized TiO2. Chemosphere 2013, 92, 925–932. [Google Scholar] [CrossRef]
- Bayan, E.M.; Pustovaya, L.E.; Volkova, M.G. Recent advances in TiO2-based materials for photocatalytic degradation of antibiotics in aqueous systems. Environ. Technol. Innov. 2021, 24, 101822. [Google Scholar] [CrossRef]
- Fakhri, A.; Behrouz, S.; Pourmand, M. Synthesis, photocatalytic and antimicrobial properties of SnO2, SnS2 and SnO2/SnS2 nanostructure. J. Photochem. Photobiol. B Biol. 2015, 149, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Qiu, J.H.; Feng, H.X.; Wang, N.X.; Sakai, E.; Komiyama, T. Preparation of MnO2/carbon nanowires composites for supercapacitors. Electrochim. Acta 2016, 212, 710–721. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, T.X.; Hua, Y.B.; Guo, X.L.; Peng, H.H.; Zhang, Y.X.; Feng, L.; Zheng, H.L. Delta manganese dioxide nanosheets decorated magnesium wire for the degradation of methyl orange. J. Colloid Interface Sci. 2017, 490, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Zhu, B.; Cheng, B.; Yu, J.; Xu, J. 2D/2D g-C3N4/MnO2 nanocomposite as a direct Z-scheme photocatalyst for enhanced photocatalytic activity. ACS Sustain. Chem. Eng. 2017, 6, 965–973. [Google Scholar] [CrossRef]
- Jung, K.W.; Lee, S.Y.; Lee, Y.J. Hydrothermal synthesis of hierarchically structured birnessite-type MnO2/biochar composites for the adsorptive removal of Cu(II) from aqueous media. Bioresour. Technol. 2018, 260, 204–212. [Google Scholar] [CrossRef]
- Marafatto, F.F.; Lanson, B.; Peña, J. Crystal growth and aggregation in suspensions of δ-MnO2 nanoparticles: Implications for surface reactivity. Environ. Sci. Nano 2018, 5, 497–508. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Meng, J.; Han, X.; Lan, Y.; Zhang, W. Past, present, and future of biochar. Biochar 2019, 1, 75–87. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Zhang, G.; Qiu, G.; Cai, D.; Wu, Z. Degradation of herbicide (glyphosate) using sunlight-sensitive MnO2/C catalyst immediately fabricated by high energy electron beam. Chem. Eng. J. 2016, 306, 696–703. [Google Scholar] [CrossRef]
- Huang, H.; Tang, J.; Gao, K.; He, R.; Zhao, H.; Werner, D. Characterization of KOH modified biochars from different pyrolysis temperatures and enhanced adsorption of antibiotics. RSC Adv. 2017, 7, 14640–14648. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xu, J.; Li, B.; Xie, Z.; Li, X.; Tang, J.; Fan, S. Enhanced adsorption performance of tetracycline in aqueous solutions by KOH modifed peanut shell derived biochar. Biomass Convers. Biorefin. 2021, 1–15. [Google Scholar] [CrossRef]
- Jia, Y.; Liu, J.; Cha, S.; Choi, S.; Park, Y.C.; Liu, C. Magnetically separable Au-TiO2/nanocube ZnFe2O4 composite for chlortetracycline removal in wastewater under visible light. J. Ind. Eng. Chem. 2017, 47, 303–314. [Google Scholar] [CrossRef]
- Liang, S.; Zhou, Y.; Kang, K.; Zhang, Y.; Cai, Z.; Pan, J. Synthesis and characterization of porous TiO2-NS/Pt/GO aerogel: A novel three-dimensional composite with enhanced visible-light photoactivity in degradation of chlortetracycline. J. Photochem. Photobiol. A Chem. 2017, 346, 1–9. [Google Scholar] [CrossRef]
- Liang, S.; Zhou, Y.; Wu, W.; Zhang, Y.; Cai, Z.; Pan, J. Preparation of porous CuO nanosheet-liked structure (CuO-NS) using C3N4 template with enhanced visible-light photoactivity in degradation of chlortetracycline. J. Photochem. Photobiol. A Chem. 2017, 346, 168–176. [Google Scholar] [CrossRef]
- Cai, T.; Liu, X.; Zhang, J.; Tie, B.; Lei, M.; Wei, X.; Peng, O.; Du, H. Silicate-modified oiltea camellia shell-derived biochar: A novel and cost-effective sorbent for cadmium removal. J. Clean. Prod. 2021, 281, 125390. [Google Scholar] [CrossRef]
- Ma, H.; Li, J.B.; Liu, W.W.; Miao, M.; Cheng, B.J.; Zhu, S.W. Novel synthesis of a versatile magnetic adsorbent derived from corncob for dye removal. Bioresour. Technol. 2015, 190, 13–20. [Google Scholar] [CrossRef]
- Wang, M.; Shen, M.; Zhang, L.; Tian, J.; Jin, X.; Zhou, Y.; Shi, J. 2D-2D MnO2/g-C3N4 heterojunction photocatalyst: In-situ synthesis and enhanced CO2 reduction activity. Carbon 2017, 120, 23–31. [Google Scholar] [CrossRef]
- Wang, D.; Guo, W.; Zhang, G.; Zhou, L.; Wang, M.; Lu, Y.; Cai, D.; Wu, Z. Remediation of Cr(VI)-contaminated acid soil using a nano composite. ACS Sustain. Chem. Eng. 2017, 5, 2246–2254. [Google Scholar] [CrossRef]
- Wang, D.F.; Dai, Z.Y.; Shu, X.; Biao, P.; Wu, L.F.; Cai, D.Q.; Wu, Z.Y. Functionalized nanocomposite for simultaneous removal of antibiotics and As(III) in swine urine aqueous solution and soil. Environ. Sci. Nano 2018, 5, 2978–2992. [Google Scholar] [CrossRef]
- Panwar, N.L.; Pawar, A. Influence of activation conditions on the physicochemical properties of activated biochar: A review. Biomass Convers. Biorefin. 2022, 12, 925–947. [Google Scholar] [CrossRef]
- Bai, Z.; Zhang, Y.; Zhang, Y.; Guo, C.; Tang, B.; Sun, D. MOFs-derived porous Mn2O3 as high-performance anode material for Li-ion battery. J. Mater. Chem. A 2015, 3, 5266–5269. [Google Scholar] [CrossRef]
- Lee, N.R.; Jung, H. Low-temperature fabrication of Mn3O4 nanorods by solid-state decomposition of exfoliated MnO2 nanosheets. J. Phys. Chem. Solids 2012, 73, 1473–1477. [Google Scholar] [CrossRef]
- Li, Q.; He, J.; Liu, D.; Yue, H.; Bai, S.; Liu, B.; Gu, L.; He, D. Facile preparation of hovenia-acerba-like hierarchical MnO2/C composites and their excellent energy storage performance for supercapacitors. J. Alloys Compd. 2017, 693, 970–978. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Y.; Chen, H.; Yang, M.; Li, H. MnO2 Nanostructures deposited on graphene-like porous carbon nanosheets for high-rate performance and high-energy density asymmetric supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 3101–3110. [Google Scholar] [CrossRef]
- Qin, J.; Qiu, F.; Rong, X.; Yan, J.; Zhao, H.; Yang, D. Adsorption behavior of crystal violet from aqueous solutions with chitosan-graphite oxide modified polyurethane as an adsorbent. J. Appl. Polym. Sci. 2015, 132, 83–101. [Google Scholar] [CrossRef]
- Shah, N.S.; Khan, J.A.; Nawaz, S.; Khan, H.M. Role of aqueous electron and hydroxyl radical in the removal of endosulfan from aqueous solution using gamma irradiation. J. Hazard. Mater. 2014, 278, 40–48. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, J.; Chen, Y.; Lu, H.; Zhuang, J.; Li, J. Synthesis of polyaniline-modified MnO2 composite nanorods and their photocatalytic application. Mater. Lett. 2014, 117, 21–23. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, H.; Wang, Z.; Qian, L.; Jin, G.; Yang, P.; Wang, D.; Xu, S.; Cai, D.; Wu, Z.; Zhang, X. Honeycomb-like MnO2/Biochar Catalyst Fabricated by High-Energy Electron Beam Irradiation for Degradation of Antibiotics in Swine Urine. Biomimetics 2023, 8, 32. https://doi.org/10.3390/biomimetics8010032
Ma H, Wang Z, Qian L, Jin G, Yang P, Wang D, Xu S, Cai D, Wu Z, Zhang X. Honeycomb-like MnO2/Biochar Catalyst Fabricated by High-Energy Electron Beam Irradiation for Degradation of Antibiotics in Swine Urine. Biomimetics. 2023; 8(1):32. https://doi.org/10.3390/biomimetics8010032
Chicago/Turabian StyleMa, Huan, Zhi Wang, Ling Qian, Gaorui Jin, Pengqi Yang, Dongfang Wang, Shengkai Xu, Dongqing Cai, Zhengyan Wu, and Xin Zhang. 2023. "Honeycomb-like MnO2/Biochar Catalyst Fabricated by High-Energy Electron Beam Irradiation for Degradation of Antibiotics in Swine Urine" Biomimetics 8, no. 1: 32. https://doi.org/10.3390/biomimetics8010032