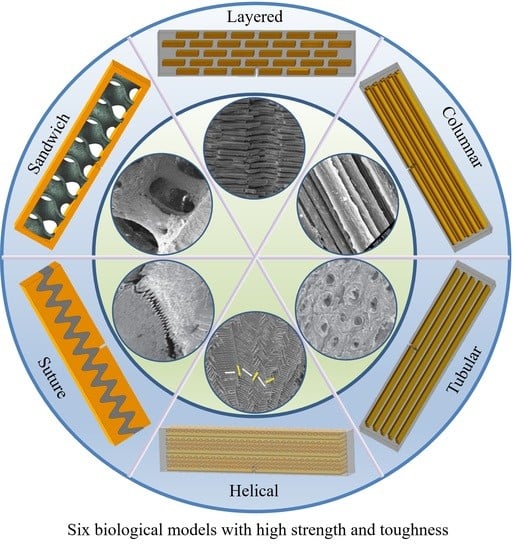
Bending Study of Six Biological Models for Design of High Strength and Tough Structures
Abstract
:1. Introduction
2. Biological Models
2.1. Layered
2.2. Columnar
2.3. Tubular
2.4. Helical
2.5. Sutured
2.6. Sandwich
3. Numerical Modelling
3.1. Simulation Setup
3.2. Simulation Results
4. Experimental Verifications
4.1. Sample Fabrications
4.2. Experimental Tests
5. Discussions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yaraghi, N.A.; Kisailus, D. Biomimetic Structural Materials: Inspiration from Design and Assembly. Annu. Rev. Phys. Chem. 2018, 69, 23–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, D. Measuring Fracture Toughness in Biological Materials. J. Mech. Behav. Biomed. Mater. 2018, 77, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Okumura, K. Strength and Toughness of Biocomposites Consisting of Soft and Hard Elements: A Few Fundamental Models. MRS Bull. 2015, 40, 333–339. [Google Scholar] [CrossRef]
- Ritchie, R.O. The Conflicts between Strength and Toughness. Nat. Mater. 2011, 10, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Launey, M.E.; Ritchie, R.O. On the Fracture Toughness of Advanced Materials. Adv. Mater. 2009, 21, 2103–2110. [Google Scholar] [CrossRef]
- Gu, G.X.; Takaffoli, M. Hierarchically Enhanced Impact Resistance of Bioinspired Composites. Adv. Mater. 2017, 29, 1700060. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, K.; Li, D.; Ji, B. Fracture Toughness of Biological Composites with Multilevel Structural Hierarchy. J. Appl. Mech. Trans. ASME 2020, 87, 071004. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Z. On the Materials Science of Nature’s Arms Race. Adv. Mater. 2018, 30, e1705220. [Google Scholar] [CrossRef]
- Wang, Y.; Naleway, S.E.; Wang, B. Biological and Bioinspired Materials: Structure Leading to Functional and Mechanical Performance. Bioact. Mater. 2020, 5, 745–757. [Google Scholar] [CrossRef]
- Beniash, E.; Stifler, C.A.; Sun, C.Y.; Jung, G.S.; Qin, Z.; Buehler, M.J.; Gilbert, P.U.P.A. The Hidden Structure of Human Enamel. Nat. Commun. 2019, 10, 4383. [Google Scholar] [CrossRef] [PubMed]
- Suksangpanya, N.; Yaraghi, N.A.; Kisailus, D.; Zavattieri, P. Twisting Cracks in Bouligand Structures. J. Mech. Behav. Biomed. Mater. 2017, 76, 38–57. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Z.; Ritchie, R.O. Interfacial Toughening Effect of Suture Structures. Acta Biomater. 2020, 102, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Restrepo, D. Multiscale Toughening Mechanisms in Biological Materials and Bioinspired Designs. Adv. Mater. 2019, 31, e1901561. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, A.; Wallin, M.; Khayyeri, H.; Isaksson, H. Crack Propagation in Cortical Bone Is Affected by the Characteristics of the Cement Line: A Parameter Study Using an XFEM Interface Damage Model. Biomech. Model. Mechanobiol. 2019, 18, 1247–1261. [Google Scholar] [CrossRef] [Green Version]
- Jia, Z.; Wang, L. 3D Printing of Biomimetic Composites with Improved Fracture Toughness. Acta Mater. 2019, 173, 61–73. [Google Scholar] [CrossRef]
- Agarwal, K. Biomimetic Tough Helicoidally Structured Material through Novel Electrospinning Based Additive Manufacturing. MRS Adv. 2019, 35, 2345–2354. [Google Scholar] [CrossRef]
- Wegst, U.G.K.; Bai, H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Bioinspired Structural Materials. Nat. Mater. 2015, 14, 23–36. [Google Scholar] [CrossRef]
- Grunenfelder, L.K.; Herrera, S. Crustacean-Derived Biomimetic Components and Nanostructured Composites. Biomim. Mater. 2014, 10, 3207–3232. [Google Scholar] [CrossRef]
- Chen, P.Y.; McKittrick, J. Biological Materials: Functional Adaptations and Bioinspired Designs. Prog. Mater. Sci. 2012, 57, 1492–1704. [Google Scholar] [CrossRef]
- Zhu, X.K.; Joyce, J.A. Review of Fracture Toughness (G, K, J, CTOD, CTOA) Testing and Standardization. Eng. Fract. Mech. 2012, 85, 1–46. [Google Scholar] [CrossRef]
- Naleway, S.E.; Porter, M.M. Structural Design Elements in Biological Materials: Application to Bioinspiration. Adv. Mater. 2015, 27, 5455–5476. [Google Scholar] [CrossRef] [Green Version]
- Libonati, F.; Buehler, M.J. Advanced Structural Materials by Bioinspiration. Adv. Eng. Mater. 2017, 19, 1600787. [Google Scholar] [CrossRef]
- Li, X.W.; Ji, H.M.; Yang, W.; Zhang, G.P.; Chen, D.L. Mechanical Properties of Crossed-Lamellar Structures in Biological Shells: A Review. J. Mech. Behav. Biomed. Mater. 2017, 74, 54–71. [Google Scholar] [CrossRef]
- Espinosa, H.D.; Rim, J.E. Merger of Structure and Material in Nacre and Bone-Perspectives on de Novo Biomimetic Materials. Prog. Mater. Sci. 2009, 54, 1059–1100. [Google Scholar] [CrossRef]
- Lenau, T.; Barfoed, M. Colours and Metallic Sheen in Beetle Shells-A Biomimetic Search for Material Structuring Principles Causing Light Interference. Adv. Eng. Mater. 2008, 10, 299–314. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.Y.; Schirer, J. Predation versus Protection: Fish Teeth and Scales Evaluated by Nanoindentation. J. Mater. Res. 2012, 27, 100–112. [Google Scholar] [CrossRef] [Green Version]
- Woesz, A.; Weaver, J.C. Micromechanical Properties of Biological Silica in Skeletons of Deep-Sea Sponges. J. Mater. Res. 2006, 21, 2068–2078. [Google Scholar] [CrossRef] [Green Version]
- Gu, G.X.; Libonati, F.; Wettermark, S.D.; Buehler, M.J. Printing Nature: Unraveling the Role of Nacre’s Mineral Bridges. J. Mech. Behav. Biomed. Mater. 2017, 76, 135–144. [Google Scholar] [CrossRef]
- Narducci, F.; Pinho, S.T. Exploiting Nacre-Inspired Crack Deflection Mechanisms in CFRP via Micro-Structural Design. Compos. Sci. Technol. 2017, 153, 178–189. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Xu, Y. Investigation of Failure Mechanisms of Nacre at Macro and Nano Scales. J. Mech. Behav. Biomed. Mater. 2020, 112, 104018. [Google Scholar] [CrossRef]
- Jiao, D.; Qu, R.T. On the Fracture Mechanisms of Nacre: Effects of Structural Orientation. J. Biomech. 2019, 96, 109336. [Google Scholar] [CrossRef]
- Bajaj, D.; Arola, D.D. On the R-Curve Behavior of Human Tooth Enamel. Biomaterials 2009, 30, 4037–4046. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Chen, C. A Strong, Tough, and Scalable Structural Material from Fast-Growing Bamboo. Adv. Mater. 2020, 32, 1906308. [Google Scholar] [CrossRef]
- Zhang, W.; Ye, C.; Zheng, K.; Zhong, J.; Tang, Y.; Fan, Y.; Buehler, M.J.; Ling, S.; Kaplan, D.L. Tensan Silk-Inspired Hierarchical Fibers for Smart Textile Applications. ACS Nano 2018, 12, 6968–6977. [Google Scholar] [CrossRef]
- Gu, Y.; Yu, L. Mechanical Properties and Application Analysis of Spider Silk Bionic Material. E-Polymers 2020, 20, 443–457. [Google Scholar] [CrossRef]
- Yeom, B.; Sain, T.; Lacevic, N.; Bukharina, D.; Cha, S.H.; Waas, A.M.; Arruda, E.M.; Kotov, N.A. Abiotic Tooth Enamel. Nature 2017, 543, 95–98. [Google Scholar] [CrossRef]
- Zou, M.; Xu, S.; Wei, C.; Wang, H.; Liu, Z. A Bionic Method for the Crashworthiness Design of Thin-Walled Structures Inspired by Bamboo. Thin-Walled Struct. 2016, 101, 222–230. [Google Scholar] [CrossRef]
- Kasapi, M.A.; Gosline, J.M. Design Complexity and Fracture Control in the Equine Hoof Wall. J. Exp. Biol. 1997, 200, 1639–1659. [Google Scholar] [CrossRef]
- Tombolato, L.; Novitskaya, E.E. Microstructure, Elastic Properties and Deformation Mechanisms of Horn Keratin. Acta Biomater. 2010, 6, 319–330. [Google Scholar] [CrossRef]
- Thompson, V.P.; Silva, N.R.F.A. Structure and Properties of Enamel and Dentin. In Non-Metallic Biomaterials for Tooth Repair and Replacement; Woodhead Publishing: Cambridge, UK, 2012; pp. 3–19. [Google Scholar]
- Wang, B.; Sullivan, T.N.; Pissarenko, A.; Zaheri, A.; Espinosa, H.D.; Meyers, M.A. Lessons from the Ocean: Whale Baleen Fracture Resistance. Adv. Mater. 2019, 31, e1804574. [Google Scholar] [CrossRef]
- Giner, E.; Belda, R.; Arango, C.; Vercher-Martínez, A.; Tarancón, J.E.; Fuenmayor, F.J. Calculation of the Critical Energy Release Rate Gc of the Cement Line in Cortical Bone Combining Experimental Tests and Finite Element Models. Eng. Fract. Mech. 2017, 184, 168–182. [Google Scholar] [CrossRef] [Green Version]
- McKittrick, J.; Chen, P.Y. Energy Absorbent Natural Materials and Bioinspired Design Strategies: A Review. Mater. Sci. Eng. C 2010, 30, 331–342. [Google Scholar] [CrossRef]
- Huang, W.; Yaraghi, N.A. A Natural Energy Absorbent Polymer Composite: The Equine Hoof Wall. Acta Biomater. 2019, 90, 267–277. [Google Scholar] [CrossRef]
- Ribbans, B.; Li, Y. A Bioinspired Study on the Interlaminar Shear Resistance of Helicoidal Fiber Structures. J. Mech. Behav. Biomed. Mater. 2016, 56, 57–67. [Google Scholar] [CrossRef]
- Suksangpanya, N.; Yaraghi, N.A.; Pipes, R.B.; Kisailus, D.; Zavattieri, P. Crack Twisting and Toughening Strategies in Bouligand Architectures. Int. J. Solids Struct. 2018, 150, 83–106. [Google Scholar] [CrossRef]
- Wu, J.; Qin, Z.; Qu, L.; Zhang, H.; Deng, F.; Guo, M. Natural Hydrogel in American Lobster: A Soft Armor with High Toughness and Strength. Acta Biomater. 2019, 88, 102–110. [Google Scholar] [CrossRef]
- Weaver, J.C.; Milliron, G.W.; Miserez, A.; Evans-Lutterodt, K.; Herrera, S.; Gallana, I.; Mershon, W.J.; Swanson, B.; Zavattieri, P.; DiMasi, E.; et al. The Stomatopod Dactyl Club: A Formidable Damage-Tolerant Biological Hammer. Science 2012, 336, 1275–1280. [Google Scholar] [CrossRef] [Green Version]
- Yin, S.; Yang, W. Hyperelastic Phase-Field Fracture Mechanics Modeling of the Toughening Induced by Bouligand Structures in Natural Materials. J. Mech. Phys. Solids 2019, 131, 204–220. [Google Scholar] [CrossRef] [Green Version]
- Sykes, D.; Hartwell, R.; Bradley, R.S.; Burnett, T.L.; Hornberger, B.; Garwood, R.J.; Withers, P.J. Time-Lapse Three-Dimensional Imaging of Crack Propagation in Beetle Cuticle. Acta Biomater. 2019, 86, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Fratzl, P.; Weinkamer, R. Nature’s Hierarchical Materials. Prog. Mater. Sci. 2007, 52, 1263–1334. [Google Scholar] [CrossRef]
- Yin, S.; Chen, H. Tough Nature-Inspired Helicoidal Composites with Printing-Induced Voids. Cell Rep. Phys. Sci. 2020, 1, 100109. [Google Scholar] [CrossRef]
- Yang, F.; Xie, W.; Meng, S. Impact and Blast Performance Enhancement in Bio-Inspired Helicoidal Structures: A Numerical Study. J. Mech. Phys. Solids 2020, 142, 104025. [Google Scholar] [CrossRef]
- Richter-Boix, A. Cranial Suture Complexity in White-Tailed Deer (Odocoileus Virginianus). J. Morphol. 2007, 849, 1042–1154. [Google Scholar]
- Yang, W.; Naleway, S.E. The Armored Carapace of the Boxfish. Acta Biomater. 2015, 23, 1–10. [Google Scholar] [CrossRef]
- Krauss, S.; Monsonego-Ornan, E.; Zelzer, E.; Fratzl, P.; Shahar, R. Mechanical Function of a Complex Three-Dimensional Suture Joining the Bony Elements in the Shell of the Red-Eared Slider Turtle. Adv. Mater. 2009, 21, 407–412. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, W.; Wang, J.; Zhang, C. Experimental and Numerical Study on Tensile Failure Behavior of Bionic Suture Joints. J. Mech. Behav. Biomed. Mater. 2019, 92, 40–49. [Google Scholar] [CrossRef]
- Rivera, J.; Hosseini, M.S. Toughening Mechanisms of the Elytra of the Diabolical Ironclad Beetle. Nature 2020, 586, 543–548. [Google Scholar] [CrossRef]
- Li, Y.; Ortiz, C. Stiffness and Strength of Suture Joints in Nature. Phys. Rev. E-Stat. Nonlinear 2011, 84, 062904. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh-Osgouei, M.; Li, Y.; Vahid, A.; Ataee, A.; Wen, C. High Strength Porous PLA Gyroid Scaffolds Manufactured via Fused Deposition Modeling for Tissue-Engineering Applications. Smart Mater. Med. 2021, 2, 15–25. [Google Scholar] [CrossRef]
- Abueidda, D.W.; Elhebeary, M.; Shiang, C.S.A.; Pang, S.; Abu Al-Rub, R.K.; Jasiuk, I.M. Mechanical Properties of 3D Printed Polymeric Gyroid Cellular Structures: Experimental and Finite Element Study. Mater. Des. 2019, 165, 107597. [Google Scholar] [CrossRef]
- Seki, Y.; Schneider, M.S.; Meyers, M.A. Structure and Mechanical Behavior of a Toucan Beak. Acta Mater. 2005, 53, 5281–5296. [Google Scholar] [CrossRef]
- Chen, P.Y.; Stokes, A.G. Comparison of the Structure and Mechanical Properties of Bovine Femur Bone and Antler of the North American Elk (Cervus Elaphus Canadensis). Acta Biomater. 2009, 5, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Meyers, M.A.; Chen, P.Y. Biological Materials: Structure and Mechanical Properties. Prog. Mater. Sci. 2008, 53, 1–206. [Google Scholar] [CrossRef] [Green Version]
- Bang, S.O.; Cho, J.U. A Study on the Compression Property of Sandwich Composite with Porous Core. Int. J. Precis. Eng. Manuf. 2015, 16, 1117–1122. [Google Scholar] [CrossRef]
- Pathipaka, R.K.; Namala, K.K.; Sunkara, N.; Bandaru, C.R. Damage Characterization of Sandwich Composites Subjected to Impact Loading. J. Sandw. Struct. Mater. 2020, 22, 2125–2138. [Google Scholar] [CrossRef]
- Yin, H.; Zheng, X.; Wen, G.; Zhang, C.; Wu, Z. Design Optimization of a Novel Bio-Inspired 3D Porous Structure for Crashworthiness. Compos. Struct. 2021, 255, 112897. [Google Scholar] [CrossRef]
- ABAQUS. SIMULIA User Assistance v2017; Dassault Systemes: Providence, RI, USA, 2017. [Google Scholar]
- Huang, Y.; Guan, Y.; Wang, L.; Zhou, J.; Ge, Z.; Hou, Y. Characterization of Mortar Fracture Based on Three Point Bending Test and XFEM. Int. J. Pavement Res. Technol. 2018, 11, 339–344. [Google Scholar] [CrossRef]
- Neto, E.A.S.; Peric, D.; Owen, D.R. Computational Methods for Plasticity: Theory and Applications; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Xie, Z.; Yao, H. Crack Deflection and Flaw Tolerance in “Brick-and-Mortar” Structured Composites. Int. J. Appl. Mech. 2014, 6, 1450017. [Google Scholar] [CrossRef]
- Standard Test Method for Determination of Reference Temperature, T0, for Ferritic Steels in the Transition Range. ASTM E1921-21a; ASTM International: West Conshohocken, PA, USA, 1998; pp. 1–20.
- Zhang, Z.; Li, Z.; Tan, Z.; Zhao, H.; Fan, G.; Xu, Y.; Xiong, D.B.; Li, Z. Bioinspired Hierarchical Al2O3/Al Laminated Composite Fabricated by Flake Powder Metallurgy. Compos. Part A Appl. Sci. Manuf. 2021, 140, 106187. [Google Scholar] [CrossRef]
- Zou, Z.; Reid, S.R.; Li, S.; Soden, P.D. Application of a Delamination Model to Laminated Composite Structures. Compos. Struct. 2002, 56, 375–389. [Google Scholar] [CrossRef]
- Kashef, S.; Asgari, A.; Hilditch, T.B.; Yan, W.; Goel, V.K.; Quadbeck, P.; Hodgson, P.D. Fracture Mechanics of Stainless Steel Foams. Mater. Sci. Eng. A 2013, 578, 115–124. [Google Scholar] [CrossRef]
- Chen, X.; Bei, G. Toughening Mechanisms in Nanolayered MAX Phase Ceramics—A Review. Materials 2017, 10, 366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montenegro, D.M.; Canal, L.P.; Botsis, J.; Zogg, M.; Studart, A.R.; Wegener, K. On the Validity of the J-Integral as a Measure of the Transverse Intralaminar Fracture Energy of Glass Fiber-Reinforced Polyurethanes with Nonlinear Material Behavior. Int. J. Solids Struct. 2018, 139–140, 15–28. [Google Scholar] [CrossRef]
- Ozaki, S.; Yamagata, K.; Ito, C.; Kohata, T.; Osada, T. Finite Element Analysis of Fracture Behavior in Ceramics: Prediction of Strength Distribution Using Microstructural Features. J. Am. Ceram. Soc. 2022, 105, 2182–2195. [Google Scholar] [CrossRef]
- Ozaki, S.; Osada, T.; Nakao, W. Finite Element Analysis of the Damage and Healing Behavior of Self-Healing Ceramic Materials. Int. J. Solids Struct. 2016, 100–101, 307–318. [Google Scholar] [CrossRef]
- Velasco-Hogan, A.; Xu, J.; Meyers, M.A. Additive Manufacturing as a Method to Design and Optimize Bioinspired Structures. Adv. Mater. 2018, 30, e1800940. [Google Scholar] [CrossRef] [PubMed]






















| Soft Matrix | Stiff Material | |
|---|---|---|
| Young’s modulus (MPa) | 600 | 3000 |
| Tensile strength (MPa) | 8.5 | 800 |
| Elongation at failure | 160% | 10% |
| Density (g/cm3) | 1.14 | 1.17 |
| Poisson ratio | 0.35 | 0.3 |
| Shore hardness | 50 | 86 |
| Soft Matrix | Stiff Material | |
|---|---|---|
| Max principal stress (MPa) | 6.5 | 350–550 |
| Displacement at failure(mm) | 0.2 | 0.01 |
| Viscosity coefficient | 0.005 | 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Lin, T.; Guo, C.; Richter, L.; Dai, N. Bending Study of Six Biological Models for Design of High Strength and Tough Structures. Biomimetics 2022, 7, 176. https://doi.org/10.3390/biomimetics7040176
Chen G, Lin T, Guo C, Richter L, Dai N. Bending Study of Six Biological Models for Design of High Strength and Tough Structures. Biomimetics. 2022; 7(4):176. https://doi.org/10.3390/biomimetics7040176
Chicago/Turabian StyleChen, Guangming, Tao Lin, Ce Guo, Lutz Richter, and Ning Dai. 2022. "Bending Study of Six Biological Models for Design of High Strength and Tough Structures" Biomimetics 7, no. 4: 176. https://doi.org/10.3390/biomimetics7040176