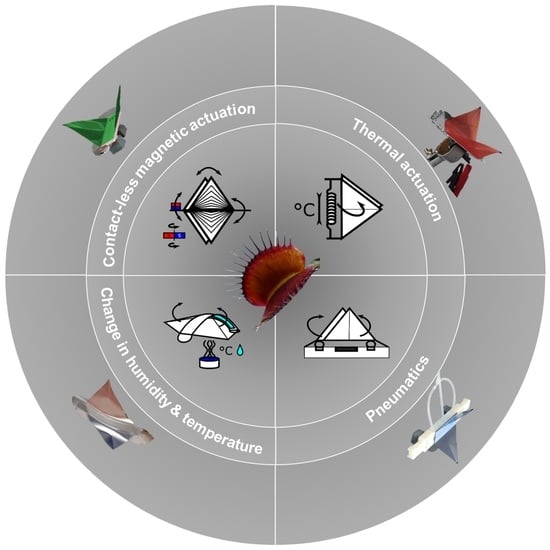
Novel Motion Sequences in Plant-Inspired Robotics: Combining Inspirations from Snap-Trapping in Two Plant Species into an Artificial Venus Flytrap Demonstrator
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setups for Movement Analysis of Dionaea Muscipula and the Artificial Venus Flyflap (VFf)
2.2. Energy Measurements
2.3. Motion Analysis of Discrete Repetitive Motion Generation by Pneumatic Actuation
2.4. Motion Analysis of Contactless Actuation of the Demonstrator by a Rotating Magnetic Field
2.5. Environmentally Triggerable Systems
2.5.1. Motion Analysis of Thermally Actuated VFf by Using SMA Springs
2.5.2. Motion Analysis in VFfs Actuated by Combination of Two Stimuli: Humidity and Temperature
2.6. Statistics
3. Results
3.1. Movement Characteristics of the Biological Model D. muscipula
3.2. Motion Analysis of Generation of Discrete Repetitive Motion by Pneumatic Actuation
3.3. Motion Analysis of Contactless Demonstrator Actuation by a Rotating Magnetic Field
3.4. Environmentally Triggerable Systems
3.4.1. Thermally Driven VFf
3.4.2. Motion Analysis in VFfs Actuated by a Combination of Two Stimuli: Humidity and Temperature
3.5. Energy and Work/Kinetic Energy Requirements and Stored Energy for and during Lobe Movement
4. Discussion
4.1. Comparison with the Biological Model
4.2. Combination of Two Snap-Trap Principles Gives a Novel Pneumatically Driven Motion Sequence
4.3. Resonance-like Movement and Generation of Contactless Fast Flapping Motion
4.4. Environmentally Triggered Motion
4.5. Overall System Discussion and Outlook
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meder, F.; Must, I.; Sadeghi, A.; Mondini, A.; Filippeschi, C.; Beccai, L.; Mattoli, V.; Pingue, P.; Mazzolai, B. Energy conversion at the cuticle of living plants. Adv. Funct. Mater. 2018, 28, 1806689. [Google Scholar] [CrossRef]
- Mazzolai, B. Plant-inspired growing robots. In Soft Robotics: Trends, Applications and Challenges; Laschi, C., Rossiter, J., Iida, F., Cianchetti, M., Margheri, L., Mazzolai, B., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Must, I.; Sinibaldi, E.; Mazzolai, B. A variable-stiffness tendril-like soft robot based on reversible osmotic actuation. Nat. Commun. 2019, 10, 344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzolai, B.; Mondini, A.; Dottore, E.D.; Sadeghi, A. Self-growing adaptable soft robots. In Mechanically Responsive Materials for Soft Robotics; Koshima, H., Ed.; Wiley-VCH: Weinheim, Germany, 2020; pp. 363–394. ISBN 3527822208. [Google Scholar]
- Mazzolai, B.; Tramacere, F.; Fiorello, I.; Margheri, L. The Bio-Engineering Approach for Plant Investigations and Growing Robots. A Mini-Review. Front. Robot. AI 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Poppinga, S.; Bauer, U.; Speck, T.; Volkov, A.G. Motile traps. In Carnivorous Plants: Physiology, Ecology, and Evolution; Ellison, A., Adamec, L., Eds.; Oxford University Press: Oxford, UK, 2018; pp. 180–193. ISBN 9780198779841. [Google Scholar]
- Sachse, R.; Westermeier, A.; Mylo, M.; Nadasdi, J.; Bischoff, M.; Speck, T.; Poppinga, S. Snapping mechanics of the Venus flytrap (Dionaea muscipula). Proc. Natl. Acad. Sci. USA 2020, 117, 16035–16042. [Google Scholar] [CrossRef]
- Poppinga, S.; Joyeux, M. Different mechanics of snap-trapping in the two closely related carnivorous plants Dionaea muscipula and Aldrovanda vesiculosa. Phys. Rev. E 2011, 84, 041928–041935. [Google Scholar] [CrossRef] [Green Version]
- Raney, J.R.; Nadkarni, N.; Daraio, C.; Kochmann, D.M.; Lewis, J.A.; Bertoldi, K. Stable propagation of mechanical signals in soft media using stored elastic energy. Proc. Natl. Acad. Sci. USA 2016, 113, 9722–9727. [Google Scholar] [CrossRef] [Green Version]
- Arrieta, A.F.; Bilgen, O.; Friswell, M.I.; Hagedorn, P. Dynamic control for morphing of bi-stable composites. J. Intell. Mater. Syst. Struct. 2013, 24, 266–273. [Google Scholar] [CrossRef]
- Kim, Y.; van den Berg, J.; Crosby, A.J. Autonomous snapping and jumping polymer gels. Nat. Mater. 2021, 20, 1695–1701. [Google Scholar] [CrossRef]
- Zhong, Y.; Du, R.; Guo, P.; Yu, H. Investigation on a new approach for designing articulated soft robots with discrete variable stiffness. IEEE/ASME Trans. Mechatron. 2021, 26, 2998–3009. [Google Scholar] [CrossRef]
- Esser, F.J.; Auth, P.; Speck, T. Artificial Venus flytraps: A research review and outlook on their importance for novel bioinspired materials systems. Front. Robot. AI 2020, 7, 75. [Google Scholar] [CrossRef]
- Pal, A.; Goswami, D.; Martinez, R.V. Elastic Energy Storage Enables Rapid and Programmable Actuation in Soft Machines. Adv. Funct. Mater. 2019, 30. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Yu, X.; Chai, H.; Li, Y.; Wu, H.; Jiang, S. Magnetic actuation bionic robotic gripper with bistable morphing structure. Compos. Struct. 2019, 229, 111422. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, D.; Wu, H.; Bao, Y.; Chai, G. Non-contact magnetic driving bioinspired Venus flytrap robot based on bistable anti-symmetric CFRP structure. Compos. Struct. 2016, 135, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Shahinpoor, M. Biomimetic robotic Venus flytrap (Dionaea muscipula Ellis) made with ionic polymer metal composites. Bioinspir. Biomim. 2011, 6, 46004. [Google Scholar] [CrossRef]
- Shi, L.; Guo, S.; Kudo, H.; Asaka, K. Development of a Venus flytrap-inspired robotic flytrap. In Proceedings of the 2012 IEEE International Conference on Robotics and Biomimetics (ROBIO), Guangzhou, China, 11–14 December 2012; pp. 551–556. [Google Scholar]
- Wani, O.M.; Zeng, H.; Priimagi, A. A light-driven artificial flytrap. Nat. Commun. 2017, 8, 15546. [Google Scholar] [CrossRef]
- Wani, O.M.; Verpaalen, R.; Zeng, H.; Priimagi, A.; Schenning, A.P.H.J. An artificial nocturnal flower via humidity-gated photoactuation in liquid crystal networks. Adv. Mater. 2018, 31, 1805985. [Google Scholar] [CrossRef]
- Lunni, D.; Cianchetti, M.; Filippeschi, C.; Sinibaldi, E.; Mazzolai, B. Plant-Inspired Soft Bistable Structures Based on Hygroscopic Electrospun Nanofibers. Adv. Mater. Interfaces 2020, 7, 1901310. [Google Scholar] [CrossRef] [Green Version]
- Esser, F.; Scherag, F.D.; Poppinga, S.; Westermeier, A.; Mylo, M.D.; Kampowski, T.; Bold, G.; Rühe, J.; Speck, T. Adaptive Biomimetic Actuator Systems Reacting to Various Stimuli by and Combining Two Biological Snap-Trap Mechanics. In Biomimetic and Biohybrid Systems, Proceedings of the 8th International Conference, Living Machines, Nara, Japan, 9–12 July 2019; Martinez-Hernandez, U., Vouloutsi, V., Mura, A., Mangan, M., Asada, M., Prescott, T.J., Verschure, P.F., Eds.; Springer: Cham, Switzerland, 2019; pp. 114–121. ISBN 978-3-030-24741-6. [Google Scholar]
- Westermeier, A.S.; Sachse, R.; Poppinga, S.; Vögele, P.; Adamec, L.; Speck, T.; Bischoff, M. How the carnivorous waterwheel plant (Aldrovanda vesiculosa) snaps. Proc. Biol. Sci. 2018, 285, 20180012. [Google Scholar] [CrossRef] [Green Version]
- Westermeier, A.; Poppinga, S.; Körner, A.; Born, L.; Sachse, R.; Saffarian, S.; Knippers, J.; Bischoff, M.; Gresser, G.T.; Speck, T. No joint ailments: How plants move and inspire technology. In Biomimetic Architecture; Knippers, J., Schmid, U., Speck, T., Eds.; Learning from Nature; Birkhäuser: Basel, Switzerland, 2019; pp. 32–41. ISBN 9783035617863. [Google Scholar]
- Esser, F.; Krüger, F.; Masselter, T.; Speck, T. Development and characterization of a novel biomimetic peristaltic pumping system with flexible silicone-based soft robotic ring actuators. In Biomimetic and Biohybrid Systems; Vouloutsi, V., Halloy, J., Mura, A., Mangan, M., Lepora, N., Prescott, T.J., Verschure, P.F.M.J., Eds.; Springer: Cham, Switzerland, 2018; pp. 157–167. ISBN 978-3-319-95971-9. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Forterre, Y.; Skotheim, J.M.; Dumais, J.; Mahadevan, L. How the Venus flytrap snaps. Nature 2005, 433, 421–425. [Google Scholar] [CrossRef]
- Jaffe, M.J. The role of ATP in mechanically stimulated rapid closure of the Venus’s flytrap. Plant Physiol. 1973, 51, 17–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poppinga, S.; Kampowski, T.; Metzger, A.; Speck, O.; Speck, T. Comparative kinematical analyses of Venus flytrap (Dionaea muscipula) snap traps. Beilstein J. Nanotechnol. 2016, 7, 664–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-W.; Koh, J.-S.; Lee, J.-G.; Ryu, J.; Cho, M.; Cho, K.-J. Flytrap-inspired robot using structurally integrated actuation based on bistability and a developable surface. Bioinspir. Biomim. 2014, 9, 36004. [Google Scholar] [CrossRef] [PubMed]
- Meder, F.; Thielen, M.; Mondini, A.; Speck, T.; Mazzolai, B. Living Plant-Hybrid Generators for Multidirectional Wind Energy Conversion. Energy Technol. 2020, 8, 2000236. [Google Scholar] [CrossRef]
- He, Q.; Wang, Z.; Song, Z.; Cai, S. Bioinspired design of vascular artificial muscle. Adv. Mater. Technol. 2019, 4, 1800244. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.-Y.; Jiang, Z.-C.; Zhao, Y. Liquid crystal polymer-based soft robots. Adv. Intell. Syst. 2020, 2, 2000148. [Google Scholar] [CrossRef]
- Kim, H.; Ahn, S.; Mackie, D.M.; Kwon, J.; Kim, S.H.; Choi, C.; Moon, Y.H.; Lee, H.B.; Ko, S.H. Shape morphing smart 3D actuator materials for micro soft robot. Mater. Today 2020, 41, 243–269. [Google Scholar] [CrossRef]
- Longo, S.J.; Cox, S.M.; Azizi, E.; Ilton, M.; Olberding, J.P.; St Pierre, R.; Patek, S.N. Beyond power amplification: Latch-mediated spring actuation is an emerging framework for the study of diverse elastic systems. J. Exp. Biol. 2019, 222, jeb197889. [Google Scholar] [CrossRef] [Green Version]
- Rendl, M.; Bönisch, A.; Mader, A.; Schuh, K.; Prucker, O.; Brandstetter, T.; Rühe, J. Simple one-step process for immobilization of biomolecules on polymer substrates based on surface-attached polymer networks. Langmuir 2011, 27, 6116–6123. [Google Scholar] [CrossRef]
- Motzki, P. Efficient SMA Actuation—Design and Control Concepts. In The 1st International Electronic Conference on Actuator Technology: Materials, Devices and Applications. The 1st International Electronic Conference on Actuator Technology: Materials, Devices and Applications. Proceedings 2020, 64, 20. [Google Scholar] [CrossRef]








| Schematic | Type | Actuation | Sensing | Snap-Buckling | Closing Time | Maximum Speed | Kinetic Energy Requirements for Actuation | Energy Consumption of the Test Setup | Efficiency of the Actuation | Reversibility |
|---|---|---|---|---|---|---|---|---|---|---|
 | Dionaea muscipula | Stimulation of trigger hairs lead to active water displacement | Touch sensitive trigger hairs | Yes | 0.15 s to 1.8 s Literature: 0.1 s to 0.5 s [27] | 0.016–0.245 m/s | Approx. 300 µmol ATP (at standard conditions equals 9.66 J) [27,28] | - | - | Yes |
 | Pneumatic VFf | Pressurized air (approx. 0.7 bar) | No sensor/actuated manually | Yes | Closing: 0.119 s to 0.311 s Opening: 0.023 s to 0.059 s | Opening movement: 3.26 m/s to 4.94 m/s | Opening: 38.49 mJ to 79.54 mJ Closing: 19.04 mJ to 30.81 mJ | 1 J for magnet valves and between 0.24 L and 0.3 L compressed air | 3.7% | Yes |
 | Magnetically driven VFf | Rotating magnetic field | No sensor/actuated manually | No | Lobes do not close completely | 0.56 m/s to 3.56 m/s | 11.52 to 17.15 mJ for manual closing | Between 27 and 39 J for the magnetic stirrer | 5.6% | Yes |
 | Thermally driven SMA VFf | Increase in temperature | Inherent to the material | No | 36.0 s to 429 s with an average of 234.7 s. | 0.000254 to 0.00117 m/s | Approx. 48.1 mJ to 315.9 mJ of energy provided by the SMA spring | 5,464,800 J for thermal heating | 3.3 × 10−8% | Yes |
 | Hydrogel coated VFf | Change in humidity | Inherent to the material | No | No real closure but unlocking time: 33.2 s to 126 s | Not determined | Environmental humidity of approx. 77% | 129,712.68 J for steam production | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tauber, F.J.; Auth, P.; Teichmann, J.; Scherag, F.D.; Speck, T. Novel Motion Sequences in Plant-Inspired Robotics: Combining Inspirations from Snap-Trapping in Two Plant Species into an Artificial Venus Flytrap Demonstrator. Biomimetics 2022, 7, 99. https://doi.org/10.3390/biomimetics7030099
Tauber FJ, Auth P, Teichmann J, Scherag FD, Speck T. Novel Motion Sequences in Plant-Inspired Robotics: Combining Inspirations from Snap-Trapping in Two Plant Species into an Artificial Venus Flytrap Demonstrator. Biomimetics. 2022; 7(3):99. https://doi.org/10.3390/biomimetics7030099
Chicago/Turabian StyleTauber, Falk J., Philipp Auth, Joscha Teichmann, Frank D. Scherag, and Thomas Speck. 2022. "Novel Motion Sequences in Plant-Inspired Robotics: Combining Inspirations from Snap-Trapping in Two Plant Species into an Artificial Venus Flytrap Demonstrator" Biomimetics 7, no. 3: 99. https://doi.org/10.3390/biomimetics7030099