Coating of Layer-by-Layer Assembly Based on Chitosan and CMC: Emerging Alternative for Quality Maintenance of Citrus Fruit
Abstract
:1. Introduction
2. Materials and Methods
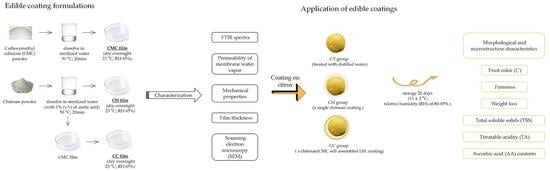
2.1. Preparation of Edible Coating Formulations
2.1.1. CMC Coating
2.1.2. Chitosan (CH) Coating
2.2. Preparation and Characterization of LbL Film
2.3. Coating on Citrus
2.3.1. Plant Materials
2.3.2. Application of Edible Coatings
2.4. Fruit Quality Attributes
2.4.1. Morphological and Microstructure Characteristics
2.4.2. Fruit Color, Firmness and Weight Loss
2.4.3. Fruit Total Soluble Solids (TSS), Titratable Acid (TA) and Ascorbic Acid (AA)
2.5. Statistical Analysis
3. Results and Discussion
3.1. Properties Characterization of the Films
3.2. Citrus Quality Attributes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Briassoulis, D.; Giannoulis, A. Evaluation of the functionality of bio-based food packaging films. Polym. Test. 2018, 69, 39–51. [Google Scholar] [CrossRef]
- Romanazzi, G.; Feliziani, E.; Baños, S.B.; Sivakumar, D. Shelf life extension of fresh fruit and vegetables by chitosan treatment. Crit. Rev. Food Sci. Nutr. 2017, 57, 579–601. [Google Scholar] [CrossRef] [PubMed]
- Elsabee, M.Z.; Abdou, E.S. Chitosan based edible films and coatings: A review. Mater. Sci. Eng. C 2013, 33, 1819–1841. [Google Scholar] [CrossRef]
- Wei, W.; Zhou, Y.-H.; Chang, H.-J.; Yeh, J.-T. Antibacterial and Miscibility Properties of Chitosan/Collagen Blends. J. Macromol. Sci. Part B 2015, 54, 143–158. [Google Scholar] [CrossRef]
- Zimet, P.; Mombrú, Á.W.; Mombrú, D.; Castro, A.; Villanueva, J.P.; Pardo, H.; Rufo, C. Physico-chemical and antilisterial properties of nisin-incorporated chitosan/carboxymethyl chitosan films. Carbohydr. Polym. 2019, 219, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, R.; Aisyah, H.; Nordin, A.; Ngadi, N.; Zuhri, M.; Asyraf, M.; Sapuan, S.; Zainudin, E.; Sharma, S.; Abral, H.; et al. Natural-Fiber-Reinforced Chitosan, Chitosan Blends and Their Nanocomposites for Various Advanced Applications. Polymers 2022, 14, 874. [Google Scholar] [CrossRef]
- Arnon, H.; Granit, R.; Porat, R.; Poverenov, E. Development of polysaccharides-based edible coatings for citrus fruits: A layer-by-layer approach. Food Chem. 2015, 166, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, C.; Aliakbarlu, J.; Cui, H.; Lin, L. Typical application of electrostatic layer-by-layer self-assembly technology in food safety assurance. Trends Food Sci. Technol. 2022, 129, 88–97. [Google Scholar] [CrossRef]
- Poverenov, E.; Rutenberg, R.; Danino, S.; Horev, B.; Rodov, V. Gelatin-Chitosan Composite Films and Edible Coatings to Enhance the Quality of Food Products: Layer-by-Layer vs. Blended Formulations. Food Bioprocess Technol. 2014, 7, 3319–3327. [Google Scholar] [CrossRef]
- Rossi-Márquez, G.; Dávalos-Saucedo, C.A.; Mayek-Pérez, N.; Di Pierro, P. Multilayered Edible Coatings to Enhance Some Quality Attributes of Ready-to-Eat Cherimoya (Annona cherimola). Coatings 2022, 13, 41. [Google Scholar] [CrossRef]
- Baswal, A.K.; Dhaliwal, H.S.; Singh, Z.; Mahajan, B.; Kalia, A.; Gill, K.S. Influence of carboxy methylcellulose, chitosan and beeswax coatings on cold storage life and quality of Kinnow mandarin fruit. Sci. Hortic. 2020, 260, 108887. [Google Scholar] [CrossRef]
- Arnon, H.; Zaitsev, Y.; Porat, R.; Poverenov, E. Effects of carboxymethyl cellulose and chitosan bilayer edible coating on postharvest quality of citrus fruit. Postharvest Biol. Technol. 2014, 87, 21–26. [Google Scholar] [CrossRef]
- Hira, N.; Mitalo, O.W.; Okada, R.; Sangawa, M.; Masuda, K.; Fujita, N.; Ushijima, K.; Akagi, T.; Kubo, Y. The effect of layer-by-layer edible coating on the shelf life and transcriptome of ‘Kosui’ Japanese pear fruit. Postharvest Biol. Technol. 2022, 185, 111787. [Google Scholar] [CrossRef]
- Leyva-Gutierrez, F.M.A.; Fei, T.; Wang, T. Synthesis of Functionalized High-Oleic Soybean Oil Wax Coatings and Emulsions for Postharvest Treatment of Fresh Citrus Fruit. Eur. J. Lipid Sci. Technol. 2020, 122, 2000005. [Google Scholar] [CrossRef]
- Saberi, B.; Golding, J.B.; Marques, J.R.; Pristijono, P.; Chockchaisawasdee, S.; Scarlett, C.J.; Stathopoulos, C.E. Application of biocomposite edible coatings based on pea starch and guar gum on quality, storability and shelf life of ‘Valencia’ oranges. Postharvest Biol. Technol. 2018, 137, 9–20. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Zhang, J.; Chen, C.; Kowaleguet, M.G.G.M.; Ban, Z.; Fei, L.; Xu, C. Chitosan-Based Layer-by-Layer Assembly: Towards Application on Quality Maintenance of Lemon Fruits. Adv. Polym. Technol. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, N.; Singh, H. Pericarp and pedicel anatomy in relation to fruit cracking in lemon (Citrus limon L Burm.). Sci. Hortic. 2019, 246, 462–468. [Google Scholar] [CrossRef]
- Van Wyk, A.A.; Huysamer, M.; Barry, G.H. Extended low-temperature shipping adversely affects rind colour of ‘Palmer Navel’ sweet orange [Citrus sinensis (L.) Osb.] due to carotenoid degradation but can partially be mitigated by optimising post-shipping holding temperature. Postharvest Biol. Technol. 2009, 53, 109–116. [Google Scholar] [CrossRef]
- Geng, P.; Chen, S.; Hu, M.; Rizwan-ul-Haq, M.; Lai, K.; Qu, F.; Zhang, Y. Combination of Kluyveromyces marxianus and sodium bicarbonate for controlling green mold of citrus fruit. Int. J. Food Microbiol. 2011, 151, 190–194. [Google Scholar] [CrossRef]
- Morales, J.; Bermejo, A.; Navarro, P.; Forner-Giner, M.Á.; Salvador, A. Rootstock effect on fruit quality, anthocyanins, sugars, hydroxycinnamic acids and flavanones content during the harvest of blood oranges ‘Moro’ and ‘Tarocco Rosso’ grown in Spain. Food Chem. 2021, 342, 128305. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Nie, W.-J. Chemical properties in fruits of mulberry species from the Xinjiang province of China. Food Chem. 2015, 174, 460–466. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, G.; Wang, Y.; Zhao, Y.; Su, H.; Tan, T. Preparation of chitosan-TiO2 composite film with efficient antimicrobial activities under visible light for food packaging applications. Carbohydr. Polym. 2017, 169, 101–107. [Google Scholar] [CrossRef]
- Nair, M.S.; Saxena, A.; Kaur, C. Effect of chitosan and alginate based coatings enriched with pomegranate peel extract to extend the postharvest quality of guava (Psidium guajava L.). Food Chem. 2018, 240, 245–252. [Google Scholar] [CrossRef]
- Liu, X.; Tang, C.; Han, W.; Xuan, H.; Ren, J.; Zhang, J.; Ge, L. Characterization and preservation effect of polyelectrolyte multilayer coating fabricated by carboxymethyl cellulose and chitosan. Colloids Surf. Physicochem. Eng. Asp. 2017, 529, 1016–1023. [Google Scholar] [CrossRef]
- Poverenov, E.; Danino, S.; Horev, B.; Granit, R.; Vinokur, Y.; Rodov, V. Layer-by-Layer Electrostatic Deposition of Edible Coating on Fresh Cut Melon Model: Anticipated and Unexpected Effects of Alginate–Chitosan Combination. Food Bioprocess Technol. 2014, 7, 1424–1432. [Google Scholar] [CrossRef]
- Shi, S.; Wang, W.; Liu, L.; Wu, S.; Wei, Y.; Li, W. Effect of chitosan/nano-silica coating on the physicochemical characteristics of longan fruit under ambient temperature. J. Food Eng. 2013, 118, 125–131. [Google Scholar] [CrossRef]
- Al-Naamani, L.; Dutta, J.; Dobretsov, S. Nanocomposite Zinc Oxide-Chitosan Coatings on Polyethylene Films for Extending Storage Life of Okra (Abelmoschus esculentus). Nanomaterials 2018, 8, 479. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Luo, Z.; Ban, Z.; Lu, H.; Li, D.; Yang, D.; Aghdam, M.S.; Li, L. The effect of the layer-by-layer (LBL) edible coating on strawberry quality and metabolites during storage. Postharvest Biol. Technol. 2019, 147, 29–38. [Google Scholar] [CrossRef]
- De Oliveira, G.A.; Bureau, S.; Renard, C.M.-G.C.; Pereira-Netto, A.B.; De Castilhos, F. Comparison of NIRS Approach for Prediction of Internal Quality Traits in Three Fruit Species. Food Chem. 2014, 143, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Ghasemnezhad, M.; Zareh, S.; Rassa, M.; Sajedi, R.H. Effect of chitosan coating on maintenance of aril quality, microbial population and PPO activity of pomegranate (Punica granatum L. cv. Tarom) at cold storage temperature: Protective effect of chitosan coating on cold-stored pomegranate. J. Sci. Food Agric. 2013, 93, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, K.S.; Gökmen, V. Extending the shelf-life of pomegranate arils with chitosan-ascorbic acid coating. LWT Food Sci. Technol. 2017, 76, 172–180. [Google Scholar] [CrossRef]
- Velickova, E.; Winkelhausen, E.; Kuzmanova, S.; Alves, V.D.; Moldão-Martins, M. Impact of chitosan-beeswax edible coatings on the quality of fresh strawberries (Fragaria ananassa cv Camarosa) under commercial storage conditions. LWT Food Sci. Technol. 2013, 52, 80–92. [Google Scholar] [CrossRef]
- Pagliarulo, C.; Sansone, F.; Moccia, S.; Russo, G.L.; Aquino, R.P.; Salvatore, P.; Di Stasio, M.; Volpe, M.G. Preservation of Strawberries with an Antifungal Edible Coating Using Peony Extracts in Chitosan. Food Bioprocess Technol. 2016, 9, 1951–1960. [Google Scholar] [CrossRef]
- Xing, Y.; Li, X.; Xu, Q.; Yun, J.; Lu, Y.; Tang, Y. Effects of chitosan coating enriched with cinnamon oil on qualitative properties of sweet pepper (Capsicum annuum L.). Food Chem. 2011, 124, 1443–1450. [Google Scholar] [CrossRef]
- Chen, C.; Peng, X.; Zeng, R.; Chen, M.; Wan, C.; Chen, J. Ficus hirta fruits extract incorporated into an alginate-based edible coating for Nanfeng mandarin preservation. Sci. Hortic. 2016, 202, 41–48. [Google Scholar] [CrossRef] [Green Version]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, C.; Liu, L.; Farouk, A.; Chen, C.; Ban, Z. Coating of Layer-by-Layer Assembly Based on Chitosan and CMC: Emerging Alternative for Quality Maintenance of Citrus Fruit. Horticulturae 2023, 9, 715. https://doi.org/10.3390/horticulturae9060715
Niu C, Liu L, Farouk A, Chen C, Ban Z. Coating of Layer-by-Layer Assembly Based on Chitosan and CMC: Emerging Alternative for Quality Maintenance of Citrus Fruit. Horticulturae. 2023; 9(6):715. https://doi.org/10.3390/horticulturae9060715
Chicago/Turabian StyleNiu, Chenyu, Lingling Liu, Amr Farouk, Cunkun Chen, and Zhaojun Ban. 2023. "Coating of Layer-by-Layer Assembly Based on Chitosan and CMC: Emerging Alternative for Quality Maintenance of Citrus Fruit" Horticulturae 9, no. 6: 715. https://doi.org/10.3390/horticulturae9060715