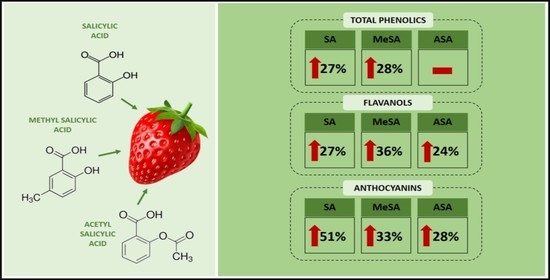
Salicylate Treatment Affects Fruit Quality and Also Alters the Composition of Metabolites in Strawberries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Plant Material
2.1.2. Chemicals
2.1.3. Fruit Quality Parameters Measurements
2.1.4. Sugars, Organic Acids and Ascorbic Acid Analysis
2.1.5. Total and Individual Phenolic Compounds Analysis
2.1.6. Statistical Analysis
3. Results
3.1. Effects of Salicylates on Fruit Colour, Firmness, Fruit Weight and Soluble Solids
3.2. Effects of Salicylates on Primary Metabolites in Strawberry Fruits
3.3. Effect of Salicylates on Secondary Metabolites in Strawberry Fruits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karlidag, H.; Yildirim, E.; Turan, M. Exogenous applications of salicylic acid affect quality and yield of strawberry grown under antifrost heated greenhouse conditions. J. Plant Nutr. Soil Sci. 2009, 172, 270–276. [Google Scholar] [CrossRef]
- Tulipani, S.; Mezzetti, B.; Capocasa, F.; Bompadre, S.; Beekwilder, J.; De Vos, C.H.R.; Capanoglu, E.; Bovy, A.; Battino, M. Antioxidants, phenolic compounds, and nutritional quality of different strawberry genotypes. J. Agric. Food Chem. 2008, 56, 696–704. [Google Scholar] [CrossRef]
- Babalar, M.; Asghari, M.; Talaei, A.; Khosroshahi, A. Effect of pre-and postharvest salicylic acid treatment on ethylene production, fungal decay and overall quality of Selva strawberry fruit. Food Chem. 2007, 105, 449–453. [Google Scholar] [CrossRef]
- Ruiz-García, Y.; Gómez-Plaza, E. Elicitors: A tool for improving fruit phenolic content. Agriculture 2013, 3, 33–52. [Google Scholar] [CrossRef] [Green Version]
- Weber, N.; Zupanc, V.; Jakopic, J.; Veberic, R.; Mikulic-Petkovsek, M.; Stampar, F. Influence of deficit irrigation on strawberry (Fragaria× ananassa Duch.) fruit quality. J. Sci. Food Agric. 2017, 97, 849–857. [Google Scholar] [CrossRef]
- Weber, N.; Veberic, R.; Rescic, J.; Mikulic-Petkovsek, M.; Stampar, F.; Koron, D.; Munda, A. Alternative products against anthracnose affect selected primary and secondary metabolites in strawberry fruit. Fruits 2016, 71, 363–371. [Google Scholar] [CrossRef] [Green Version]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Weber, N.; Veberic, R.; Stampar, F.; Munda, A.; Koron, D. Alteration of the content of primary and secondary metabolites in strawberry fruit by Colletotrichum nymphaeae infection. J. Agric. Food Chem. 2013, 61, 5987–5995. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Slatnar, A.; Stampar, F.; Veberic, R. HPLC–MSn identification and quantification of flavonol glycosides in 28 wild and cultivated berry species. Food Chem. 2012, 135, 2138–2146. [Google Scholar] [CrossRef] [PubMed]
- Kårlund, A.; Salminen, J.-P.; Koskinen, P.; Ahern, J.R.; Karonen, M.; Tiilikkala, K.; Karjalainen, R.O. Polyphenols in strawberry (Fragaria× ananassa) leaves induced by plant activators. J. Agric. Food Chem. 2014, 62, 4592–4600. [Google Scholar] [CrossRef]
- Liu, J.; Tian, S.; Meng, X.; Xu, Y. Effects of chitosan on control of postharvest diseases and physiological responses of tomato fruit. Postharvest Biol. Technol. 2007, 44, 300–306. [Google Scholar] [CrossRef]
- Heredia, J.B.; Cisneros-Zevallos, L. The effects of exogenous ethylene and methyl jasmonate on the accumulation of phenolic antioxidants in selected whole and wounded fresh produce. Food Chem. 2009, 115, 1500–1508. [Google Scholar] [CrossRef]
- Shafiee, M.; Taghavi, T.S.; Babalar, M. Addition of salicylic acid to nutrient solution combined with postharvest treatments (hot water, salicylic acid, and calcium dipping) improved postharvest fruit quality of strawberry. Sci. Hortic. 2010, 124, 40–45. [Google Scholar] [CrossRef]
- Kazemi, M. Foliar application of salicylic acid and calcium on yield, yield component and chemical properties of strawberry. Bull. Env. Pharmacol. Life Sci. 2013, 2, 19–23. [Google Scholar]
- Peng, L.; Jiang, Y. Exogenous salicylic acid inhibits browning of fresh-cut Chinese water chestnut. Food Chem. 2006, 94, 535–540. [Google Scholar] [CrossRef]
- Kumar, D. Salicylic acid signaling in disease resistance. Plant Sci. 2014, 228, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Raskin, I. Salicylate, a new plant hormone. Plant Physiol. 1992, 99, 799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazar, R.; Iqbal, N.; Khan, N.A. Salicylic Acid: A Multifaceted Hormone; Springer: Berlin, Germany, 2017; ISBN 9811060681. [Google Scholar]
- Mitchell, A.G.; Broadhead, J.F. Hydrolysis of solubilized aspirin. J. Pharm. Sci. 1967, 56, 1261–1266. [Google Scholar] [CrossRef]
- Mo, Y.; Gong, D.; Liang, G.; Han, R.; Xie, J.; Li, W. Enhanced preservation effects of sugar apple fruits by salicylic acid treatment during post-harvest storage. J. Sci. Food Agric. 2008, 88, 2693–2699. [Google Scholar] [CrossRef]
- Giménez, M.J.; Serrano, M.; Valverde, J.M.; Martínez-Romero, D.; Castillo, S.; Valero, D.; Guillén, F. Preharvest salicylic acid and acetylsalicylic acid treatments preserve quality and enhance antioxidant systems during postharvest storage of sweet cherry cultivars. J. Sci. Food Agric. 2017, 97, 1220–1228. [Google Scholar] [CrossRef]
- Giménez, M.J.; Valverde, J.M.; Valero, D.; Guillén, F.; Martínez-Romero, D.; Serrano, M.; Castillo, S. Quality and antioxidant properties on sweet cherries as affected by preharvest salicylic and acetylsalicylic acids treatments. Food Chem. 2014, 160, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Halitschke, R.; Yin, C.; Liu, C.-J.; Gan, S.-S. Salicylic acid 3-hydroxylase regulates Arabidopsis leaf longevity by mediating salicylic acid catabolism. Proc. Natl. Acad. Sci. USA 2013, 110, 14807–14812. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Esplá, A.; Serrano, M.; Valero, D.; Martínez-Romero, D.; Castillo, S.; Zapata, P.J. Enhancement of antioxidant systems and storability of two plum cultivars by preharvest treatments with salicylates. Int. J. Mol. Sci. 2017, 18, 1911. [Google Scholar] [CrossRef]
- Dieryckx, C.; Gaudin, V.; Dupuy, J.-W.; Bonneu, M.; Girard, V.; Job, D. Beyond plant defense: Insights on the potential of salicylic and methylsalicylic acid to contain growth of the phytopathogen Botrytis cinerea. Front. Plant Sci. 2015, 6, 859. [Google Scholar] [CrossRef] [Green Version]
- García-Pastor, M.E.; Zapata, P.J.; Castillo, S.; Martínez-Romero, D.; Guillén, F.; Valero, D.; Serrano, M. The effects of salicylic acid and its derivatives on increasing pomegranate fruit quality and bioactive compounds at harvest and during storage. Front. Plant Sci. 2020, 11, 668. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.A.; Abdelbaset, A.-K.; Abd-Elkader, D.Y. Salicylic acid effects on growth, yield, and fruit quality of strawberry cultivars. J. Med. Act. Plants 2018, 6, 1–11. [Google Scholar]
- Lu, X.; Sun, D.; Li, Y.; Shi, W.; Sun, G. Pre-and post-harvest salicylic acid treatments alleviate internal browning and maintain quality of winter pineapple fruit. Sci. Hortic. 2011, 130, 97–101. [Google Scholar] [CrossRef]
- Perkins-Veazie, P. Growth and ripening of strawberry fruit. Hortic. Rev. 1995, 17, 267–297. [Google Scholar]
- Alrashdi, A.M.A.; Al-Qurashi, A.D.; Awad, M.A.; Mohamed, S.A.; Al-rashdi, A.A. Quality, antioxidant compounds, antioxidant capacity and enzymes activity of ‘El-Bayadi’table grapes at harvest as affected by preharvest salicylic acid and gibberellic acid spray. Sci. Hortic. 2017, 220, 243–249. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. Composition of sugars, organic acids, and total phenolics in 25 wild or cultivated berry species. J. Food Sci. 2012, 77, C1064–C1070. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.; Ghosh, U. Effect of Thermal Treatment on Ascorbic Acid Content of Pomegranate Juice; NISCAIR-CSIR: India, New Delhi, 2012; pp. 309–312. [Google Scholar]
- Quaglia, G.B.; Gravina, R.; Paperi, R.; Paoletti, F. Effect of high pressure treatments on peroxidase activity, ascorbic acid content and texture in green peas. LWT-Food Sci. Technol. 1996, 29, 552–555. [Google Scholar] [CrossRef]
- Akhtar, A.; Abbasi, N.A.; Hussain, A. Effect of calcium chloride treatments on quality characteristics of loquat fruit during storage. Pakistan J. Bot. 2010, 42, 181–188. [Google Scholar]
- Aghaeifard, F.; Babalar, M.; Fallahi, E.; Ahmadi, A. Influence of humic acid and salicylic acid on yield, fruit quality, and leaf mineral elements of strawberry (Fragaria× Ananassa duch.) cv. Camarosa. J. Plant Nutr. 2016, 39, 1821–1829. [Google Scholar] [CrossRef]
- Huang, R.-H.; Liu, J.-H.; Lu, Y.-M.; Xia, R.-X. Effect of salicylic acid on the antioxidant system in the pulp of ‘Cara cara’navel orange (Citrus sinensis L. Osbeck) at different storage temperatures. Postharvest Biol. Technol. 2008, 47, 168–175. [Google Scholar] [CrossRef]
- Mazurek, A.; Pankiewicz, U. Changes of dehydroascorbic acid content in relation to total content of vitamin C in selected fruits and vegetables. Acta Sci. Pol. Hortorum Cultus 2012, 11, 169–177. [Google Scholar]
- Wiśniewska, H.; Chełkowski, J. Influence of exogenic salicylic acid on Fusarium seedling blight reduction in barley. Acta Physiol. Plant. 1999, 21, 63–66. [Google Scholar] [CrossRef]
- Ho, C.-T. Phenolic Compounds in Food: An Overview; The State University of New Jersey: New Brunswick, NJ, USA, 1992; pp. 1–7. [Google Scholar]
- Weber, N.; Veberic, R.; Mikulic-Petkovsek, M.; Stampar, F.; Koron, D.; Munda, A.; Jakopic, J. Metabolite accumulation in strawberry (Fragaria× ananassa Duch.) fruits and runners in response to Colletotrichum nymphaeae infection. Physiol. Mol. Plant Pathol. 2015, 92, 119–129. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, L.; Zhang, X.; Xu, L.; Cao, J.; Jiang, W. The effect of exogenous salicylic acid on antioxidant activity, bioactive compounds and antioxidant system in apricot fruit. Sci. Hortic. 2015, 181, 113–120. [Google Scholar] [CrossRef]
- Gacnik, S.; Veberic, R.; Marinovic, S.; Halbwirth, H.; Mikulic-Petkovsek, M. Effect of pre-harvest treatments with salicylic and methyl salicylic acid on the chemical profile and activity of some phenylpropanoid pathway related enzymes in apple leaves. Sci. Hortic. 2021, 277, 109794. [Google Scholar] [CrossRef]
- Li, Z.; Wang, N.; Wei, Y.; Zou, X.; Jiang, S.; Xu, F.; Wang, H.; Shao, X. Terpinen-4-ol enhances disease resistance of postharvest strawberry fruit more effectively than tea tree oil by activating the phenylpropanoid metabolism pathway. J. Agric. Food Chem. 2020, 68, 6739–6747. [Google Scholar] [CrossRef]
- Li, Z.; Wei, Y.; Cao, Z.; Jiang, S.; Chen, Y.; Shao, X. The Jasmonic Acid Signaling Pathway is Associated with Terpinen-4-ol-Induced Disease Resistance against Botrytis cinerea in Strawberry Fruit. J. Agric. Food Chem. 2021, 36, 10678–10687. [Google Scholar] [CrossRef]
- Blanch, G.P.; Gómez-Jiménez, M.C.; Del Castillo, M.L.R. Exogenous salicylic acid improves phenolic content and antioxidant activity in table grapes. Plant Foods Hum. Nutr. 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Preciado-Rangel, P.; Reyes-Pérez, J.J.; Ramírez-Rodríguez, S.C.; Salas-Pérez, L.; Fortis-Hernández, M.; Murillo-Amador, B.; Troyo-Diéguez, E. Foliar aspersion of salicylic acid improves phenolic and flavonoid compounds, and also the fruit yield in cucumber (Cucumis sativus L.). Plants 2019, 8, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tareen, M.J.; Abbasi, N.A.; Hafiz, I.A. Effect of salicylic acid treatments on storage life of peach fruits cv. ‘Flordaking.’ Pakistan J. Bot. 2012, 44, 119–124. [Google Scholar]
- El-Mogy, M.M.; Ali, M.R.; Darwish, O.S.; Rogers, H.J. Impact of salicylic acid, abscisic acid, and methyl jasmonate on postharvest quality and bioactive compounds of cultivated strawberry fruit. J. Berry Res. 2019, 9, 333–348. [Google Scholar] [CrossRef]
- Hosseinifarahi, M.; Jamshidi, E.; Amiri, S.; Kamyab, F.; Radi, M. Quality, phenolic content, antioxidant activity, and the degradation kinetic of some quality parameters in strawberry fruit coated with salicylic acid and Aloe vera gel. J. Food Process. Preserv. 2020, 44, e14647. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.E.; Karimi, E. Involvement of salicylic acid on antioxidant and anticancer properties, anthocyanin production and chalcone synthase activity in ginger (Zingiber officinale Roscoe) varieties. Int. J. Mol. Sci. 2012, 13, 14828–14844. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Wan, G.; Liang, Z. Accumulation of salicylic acid-induced phenolic compounds and raised activities of secondary metabolic and antioxidative enzymes in Salvia miltiorrhiza cell culture. J. Biotechnol. 2010, 148, 99–104. [Google Scholar] [CrossRef]
- Obinata, N.; Yamakawa, T.; Takamiya, M.; TANAKA, N.; ISHIMARU, K.; KODAMA, T. Effects of salicylic acid on the production of procyanidin and anthocyanin in cultured grape cells. Plant Biotechnol. 2003, 20, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Sudha, G.; Ravishankar, G.A. Elicitation of anthocyanin production in callus cultures of Daucus carota and the involvement of methyl jasmonate and salicylic acid. Acta Physiol. Plant. 2003, 25, 249–256. [Google Scholar] [CrossRef]


| Treatment | L* | C | h° | Fruit Firmness (kg/cm2) | Soluble Solids (°Brix) | Fruit Weight (g) | |
|---|---|---|---|---|---|---|---|
| T0 | C | 33.54 ± 0.52 a | 40.03 ± 0.94 a | 31.15 ± 0.63 a | 0.79 ± 0.09 a | 7.89 ± 0.30 a | 13.21 ± 0.75 a |
| SA | 33.93 ± 0.64 a | 41.47 ± 0.87 a | 31.24 ± 0.58 a | 0.88 ± 0.09 a | 7.18 ± 0.23 a | 12.03 ± 0.86 a | |
| MeSA | 33.16 ± 0.55 a | 39.17 ± 1.09 a | 31.11 ± 0.98 a | 0.98 ± 0.11 a | 7.43 ± 0.28 a | 11.55 ± 0.45 a | |
| ASA | 33.14 ± 0.45 a | 41.56 ± 0.93 a | 31.97 ± 0.56 a | 0.98 ± 0.09 a | 7.99 ± 0.24 a | 12.86 ± 0.51 a | |
| 24 h | C | 32.74 ± 2.18 a | 42.25 ± 1.21 a | 31.46 ± 0.27 b | 0.73 ± 0.04 a | 7.57 ± 0.19 a | 13.05 ± 0.74 a |
| SA | 34.37 ± 0.70 a | 41.23 ± 1.00 a | 27.45 ± 0.69 a | 1.07 ± 0.08 b | 8.97 ± 0.23 b | 13.78 ± 0.66 a | |
| MeSA | 33.42 ± 0.63 a | 39.27 ± 1.26 a | 28.77 ± 0.52 a | 0.94 ± 0.04 b | 7.91 ± 0.26 a | 13.64 ± 0.80 a | |
| ASA | 33.71 ± 0.54 a | 40.92 ± 1.69 a | 28.02 ± 0.76 a | 0.88 ± 0.04 ab | 7.88 ± 0.32 a | 14.07 ± 0.91 a | |
| 72 h | C | 34.90 ± 0.6 ab | 42.00 ± 0.94 a | 32.69 ± 0.30 d | 0.49 ± 0.04 ab | 8.66 ± 0.34 a | 12.45 ± 0.72 a |
| SA | 34.67 ± 0.53 ab | 42.81 ± 0.78 a | 27.15 ± 0.29 a | 0.66 ± 0.03 c | 9.07 ± 0.21 a | 12.99 ± 0.75 a | |
| MeSA | 33.20 ± 0.58 a | 40.66 ± 0.82 a | 28.80 ± 0.44 b | 0.40 ± 0.03 a | 8.53 ± 0.35 a | 13.08 ± 0.69 a | |
| ASA | 35.31 ± 0.40 b | 42.43 ± 0.76 a | 30.46 ± 0.54 c | 0.53 ± 0.03 bc | 8.59 ± 0.34 a | 15.08 ± 0.9 a | |
| 7 days | C | 33.33 ± 0.49 a | 37.43 ± 1.20 a | 29.32 ± 0.37 b | 0.45 ± 0.03 a | 8.97 ± 0.47 a | 8.54 ± 0.74 ab |
| SA | 34.09 ± 0.67 a | 38.58 ± 1.12 a | 27.25 ± 0.52 a | 0.34 ± 0.04 a | 10.49 ± 0.44 a | 8.60 ± 0.38 b | |
| MeSA | 32.63 ± 0.49 a | 35.60 ± 1.05 a | 26.28 ± 0.55 a | 0.33 ± 0.03 a | 8.82 ± 0.50 a | 7.48 ± 0.50 a | |
| ASA | 32.63 ± 0.71 a | 36.80 ± 1.40 a | 26.81 ± 0.70 a | 0.33 ± 0.03 a | 8.90 ± 0.36 a | 8.60 ± 0.66 ab | |
| 10 day | C | 32.97 ± 0.62 a | 36.13 ± 1.22 a | 29.65 ± 0.39 b | 0.25 ± 0.02 a | 8.57 ± 0.47 a | 7.23 ± 0.48 a |
| SA | 33.21 ± 0.67 a | 36.81 ± 1.05 a | 26.69 ± 0.55 a | 0.39 ± 0.04 b | 9.13 ± 0.48 a | 6.99 ± 0.37 a | |
| MeSA | 32.30 ± 0.85 a | 34.15 ± 1.66 a | 26.83 ± 0.51 a | 0.45 ± 0.04 b | 8.57 ± 0.38 a | 6.20 ± 0.31 a | |
| ASA | 32.70 ± 0.46 a | 36.03 ± 0.95 a | 27.19 ± 0.27 a | 0.32 ± 0.05 ab | 9.17 ± 0.47 a | 7.16 ± 0.39 a |
| Treatment | Sucrose | Glucose | Fructose | Total Sugars a | Citric | Malic | Fumaric | Total Acids b | Ascorbic Acid | |
|---|---|---|---|---|---|---|---|---|---|---|
| T0 | C | 6.70 ± 1.09 a | 21.38 ± 1.23 a | 21.76 ± 1.25 a | 49.81 ± 3.53 a | 6.77 ± 0.26 a | 0.53 ± 0.03 a | 0.40 ± 0.02 a | 7.70 ± 0.24 a | 38.34 ± 1.65 a |
| SA | 5.45 ± 0.92 a | 21.15 ± 1.01 a | 21.49 ± 1.03 a | 48.1 ± 2.85 a | 6.76 ± 0.62 a | 0.52 ± 0.03 a | 0.39 ± 0.02 a | 7.67 ± 0.62 a | 40.22 ± 2.05 a | |
| MeSA | 6.47 ± 0.75 a | 22.59 ± 1.22 a | 22.95 ± 1.24 a | 52.01 ± 3.06 a | 6.46 ± 0.63 a | 0.53 ± 0.02 a | 0.40 ± 0.02 a | 7.41 ± 0.63 a | 42.55 ± 4.58 a | |
| ASA | 7.39 ± 0.84 a | 22.58 ± 0.84 a | 22.94 ± 0.85 a | 52.92 ± 2.43 a | 7.30 ± 0.33 a | 0.53 ± 0.04 a | 0.40 ± 0.03 a | 8.24 ± 0.36 a | 41.57 ± 1.96 a | |
| 24 h | C | 5.11 ± 0.58 a | 16.64 ± 0.55 a | 18.17 ± 0.58 a | 39.91 ± 1.11 a | 5.13 ± 0.25 a | 0.39 ± 0.04 a | 0.35 ± 0.02 a | 7.51 ± 0.35 b | 33.97 ± 1.05 a |
| SA | 6.71 ± 0.21 a | 21.27 ± 0.80 b | 22.41 ± 0.45 c | 50.39 ± 1.05 b | 6.61 ± 0.32 b | 0.51 ± 0.02 b | 0.39 ± 0.02 a | 5.88 ± 0.26 a | 41.06 ± 1.32 b | |
| MeSA | 6.77 ± 0.67 a | 19.63 ± 0.32 ab | 19.95 ± 0.33 ab | 46.34 ± 1.20 ab | 6.00 ± 0.25 ab | 0.47 ± 0.03 ab | 0.36 ± 0.02 a | 6.84 ± 0.28 ab | 38.03 ± 0.84 ab | |
| ASA | 7.53 ± 1.28 a | 20.50 ± 1.21 b | 21.64 ± 0.53 bc | 49.67 ± 2.96 b | 6.41 ± 0.15 b | 0.52 ± 0.03 b | 0.39 ± 0.02 a | 7.31 ± 0.16 b | 37.86 ± 0.92 ab | |
| 72 h | C | 8.55 ± 0.52 a | 22.93 ± 0.46 a | 22.52 ± 0.36 a | 54.00 ± 1.02 a | 5.95 ± 0.26 ab | 0.48 ± 0.03 a | 0.36 ± 0.02 a | 8.12 ± 0.27 b | 37.69 ± 0.91 a |
| SA | 10.86 ± 0.46 bc | 26.03 ± 0.20 b | 26.46 ± 0.21 c | 63.35 ± 0.59 b | 7.22 ± 0.27 b | 0.51 ± 0.02 a | 0.38 ± 0.02 a | 6.79 ± 0.30 a | 43.83 ± 1.52 b | |
| MeSA | 9.58 ± 0.57 ab | 24.79 ± 0.74 ab | 24.19 ± 0.74 ab | 58.57 ± 1.80 ab | 6.13 ± 0.36 ab | 0.48 ± 0.02 a | 0.36 ± 0.01 a | 6.97 ± 0.36 ab | 38.40 ± 1.46 ab | |
| ASA | 12.16 ± 0.69 c | 24.32 ± 0.54 ab | 25.10 ± 0.47 bc | 61.58 ± 1.53 b | 5.66 ± 0.37 a | 0.52 ± 0.03 a | 0.39 ± 0.02 a | 6.57 ± 0.35 a | 38.49 ± 1.88 ab | |
| 7 days | C | 6.49 ± 0.51 a | 22.11 ± 1.02 a | 21.86 ± 2.25 a | 50.47 ± 2.44 a | 5.88 ± 0.44 a | 0.4 ± 0.02 a | 0.30 ± 0.02 a | 6.59 ± 0.45 a | 43.44 ± 3.01 a |
| SA | 10.61 ± 0.63 b | 25.42 ± 1.59 a | 25.82 ± 1.62 a | 61.85 ± 3.01 a | 5.46 ± 0.49 a | 0.46 ± 0.04 a | 0.34 ± 0.03 a | 6.26 ± 0.55 a | 42.99 ± 2.21 a | |
| MeSA | 10.55 ± 0.86 b | 23.69 ± 2.00 a | 24.08 ± 2.03 a | 58.32 ± 4.20 a | 5.79 ± 0.33 a | 0.43 ± 0.03 a | 0.32 ± 0.02 a | 6.54 ± 0.33 a | 42.80 ± 1.38 a | |
| ASA | 9.06 ± 0.47 b | 23.36 ± 1.62 a | 23.74 ± 1.64 a | 56.16 ± 3.57 a | 4.94 ± 0.40 a | 0.42 ± 0.02 a | 0.32 ± 0.01 a | 5.68 ± 0.42 a | 39.09 ± 2.11 a | |
| 10 days | C | 6.97 ± 0.55 a | 19.10 ± 0.78 a | 23.70 ± 3.4 a | 49.08 ± 1.03 ab | 5.23 ± 0.29 a | 0.45 ± 0.04 a | 0.34 ± 0.03 a | 6.02 ± 0.31 a | 41.16 ± 1.60 a |
| SA | 7.26 ± 0.97 a | 23.57 ± 0.89 b | 24.13 ± 0.94 a | 54.96 ± 2.27 bc | 6.44 ± 0.39 a | 0.29 ± 0.02 a | 0.29 ± 0.02 a | 7.11 ± 0.39 a | 49.44 ± 1.19 b | |
| MeSA | 9.09 ± 0.48 a | 24.79 ± 0.42 b | 25.20 ± 0.43 a | 59.08 ± 1.03 c | 6.08 ± 0.50 a | 0.31 ± 0.02 a | 0.31 ± 0.02 a | 6.82 ± 0.48 a | 49.38 ± 2.78 b | |
| ASA | 7.34 ± 0.34 a | 18.61 ± 0.67 a | 18.10 ± 0.66 a | 44.06 ± 0.94 a | 4.97 ± 0.35 a | 0.29 ± 0.01 a | 0.29 ± 0.01 a | 5.66 ± 0.36 a | 40.13 ± 2.17 a |
| Peak No | Identified Phenolic Compounds | [M-H]− (m/z) | MS2 (m/z) | Phenolic Group |
|---|---|---|---|---|
| 1 | bis HHDP hexose 1 | 783 | 481,301 | EAD |
| 2 | Cyanidin-3-glucoside | 449 | 287 | ANT |
| 3 | bis HHDP hexose 2 | 783 | 481,301 | EAD |
| 4 | Procyanidin dimer 1 | 577 | 425,407,289 | FLA |
| 5 | Pelargonidin-3-glucoside | 433 | 271 | ANT |
| 6 | Pelargonidin-3-rutinoside | 579 | 271 | ANT |
| 7 | Procyanidin dimer 2 | 577 | 425,407,289 | FLA |
| 8 | Chlorogenic acid | 353 | 191,179 | HCAD |
| 8 | Catechin | 289 | 245 | FLA |
| 9 | p-coumaric acid hexoside | 325 | 163,119 | HCAD |
| 10 | Caffeic acid derivative | 335 | 179,135 | HCAD |
| 11 | Cyanidin-3-malonylglucoside | 535 | 287 | ANT |
| 12 | Pelargonidin-3-malonylglucoside | 519 | 271 | ANT |
| 13 | Cinamoyl hexoside | 355 | 309,207,147 | HCAD |
| 14 | Apigenin rhamnoside | 415 | 269 | FVN |
| 15 | Apigenin acetyl hexoside 1 | 473 | 269 | FVN |
| 16 | Apigenin acetyl hexoside 2 | 473 | 269 | FVN |
| 16 | Eriodictyol acetyl hexoside | 491 | 287 | FLV |
| 17 | Ellagic acid pentoside 1 | 433 | 301 | EAD |
| 18 | Ellagic acid hexoside | 463 | 301 | EAD |
| 19 | Naringenin hexoside | 433 | 271 | FLV |
| 20 | Ellagic acid rhamnoside | 447 | 301 | EAD |
| 21 | Ellagic acid deoxy hexoside | 447 | 301 | EAD |
| 22 | Ellagic acid pentoside 2 | 433 | 301 | EAD |
| 23 | Quercetin-pentose hexoside | 595 | 301 | FLO |
| 24 | Quercetin-3-rutinoside | 609 | 301 | FLO |
| 25 | Quercetin-3-glucoside | 463 | 301 | FLO |
| 26 | Kaempferol-3-rutinoside | 593 | 285 | FLO |
| 27 | Quercetin-3-glucuronide | 477 | 301 | FLO |
| 28 | Kaempferol-3-glucoside | 447 | 285 | FLO |
| 29 | Isorhamnetin hexoside | 477 | 315 | FLO |
| 30 | Kaempferol-3-glucuronide | 461 | 285 | FLO |
| 31 | Kaempferol acetyl hexoside | 489 | 285 | FLO |
| 32 | Kaempferol-3-coumaroyl glucoside | 593 | 285 | FLO |
| Treatment | Hydroxycinn-Amic Acid Derivatives b | Flavonols a | Flavanols a | Flavanones a | Flavones a | Ellagic Acid Derivatives a | Anthocyanins a | Total Phenolics b | |
|---|---|---|---|---|---|---|---|---|---|
| T0 | C | 174.39 ± 6.72 a | 11.19 ± 1.44 a | 354.81 ± 10.20 a | 1.14 ± 0.09 a | 114.48 ± 1.80 a | 116.37 ± 7.35 a | 386.73 ± 8.37 a | 1169.52 ± 11.37 a |
| SA | 184.80 ± 12.15 a | 10.23 ± 0.93 a | 349.80 ± 2.28 a | 1.08 ± 0.21 a | 106.53 ± 2.79 a | 117.54 ± 8.43 a | 392.07 ± 13.62 a | 1171.11 ± 18.90 a | |
| MeSA | 185.55 ± 8.88 a | 9.81 ± 1.83 a | 345.66 ± 20.61 a | 1.23 ± 0.18 a | 109.80 ± 2.46 a | 118.65 ± 6.00 a | 406.50 ± 16.20 a | 1170.20 ± 41.31 a | |
| ASA | 180.72 ± 4.26 a | 10.74 ± 2.31 a | 363.15 ± 16.74 a | 0.99 ± 0.15 a | 112.53 ± 4.74 a | 107.04 ± 7.98 a | 373.50 ± 12.09 a | 1160.49 ± 32.4 a | |
| 24 h | C | 184.59 ± 1.74 a | 6.21 ± 0.33 a | 310.47 ± 10.53 a | 1.26 ± 0.12 a | 110.55 ± 2.67 a | 115.68 ± 9.18 a | 298.80 ± 2.37 a | 1040.10 ± 18.72 a |
| SA | 215.76 ± 1.77 c | 11.70 ± 0.87 b | 396.90 ± 8.91 c | 1.05 ± 0.18 a | 129.54 ± 2.73 b | 111.81 ± 10.83 a | 450.06 ± 7.59 d | 1323.63 ± 17.07 c | |
| MeSA | 197.97 ± 2.55 b | 10.83 ± 1.50 b | 373.02 ± 5.40 bc | 1.32 ± 0.12 a | 129.63 ± 5.34 b | 115.98 ± 7.74 a | 333.60 ± 6.42 b | 1172.31 ± 17.49 b | |
| ASA | 185.22 ± 1.62 a | 10.56 ± 0.57 b | 335.25 ± 17.16 ab | 1.20 ± 0.18 a | 129.54 ± 3.93 b | 111.39 ± 14.88 a | 372.03 ± 9.54 c | 1155.48 ± 30.84 b | |
| 72 h | C | 220.53 ± 6.51 a | 6.39 ± 0.99 a | 382.71 ± 17.01 a | 1.08 ± 0.12 a | 108.15 ± 7.62 a | 126.36 ± 11.76 a | 352.83 ± 8.16 a | 1204.26 ± 64.41 a |
| SA | 253.17 ± 7.92 b | 13.86 ± 0.66 b | 482.85 ± 11.55 b | 1.14 ± 0.15 a | 116.40 ± 5.64 a | 136.44 ± 3.36 a | 436.83 ± 14.25 bc | 1476.18 ± 31.47 bc | |
| MeSA | 250.65 ± 5.73 ab | 14.94 ± 1.68 b | 518.70 ± 21.15 b | 1.41 ± 0.18 a | 156.51 ± 12.60 b | 150.72 ± 8.67 a | 469.83 ± 14.25 c | 1546.62 ± 107.55 c | |
| ASA | 236.49 ± 9.78 ab | 9.18 ± 0.84 a | 474.99 ± 13.71 b | 1.05 ± 0.15 a | 107.43 ± 5.28 a | 121.77 ± 8.16 a | 392.91 ± 12.27 ab | 1364.37 ± 33.51 b | |
| 7 days | C | 255.18 ± 13.92 a | 8.46 ± 1.20 a | 587.01 ± 12.27 ab | 1.74 ± 0.27 a | 156.87 ± 11.58 a | 180.93 ± 14.07 a | 542.28 ± 15.75 a | 1749.42 ± 41.85 a |
| SA | 301.71 ± 5.73 b | 15.69 ± 2.40 a | 591.33 ± 20.34 ab | 1.92 ± 0.39 a | 155.13 ± 13.71 a | 169.11 ± 14.46 a | 608.91 ± 16.56 ab | 1851.06 ± 55.38 a | |
| MeSA | 229.17 ± 7.56 a | 12.48 ± 2.52 a | 581.13 ± 4.98 a | 2.04 ± 0.39 a | 162.78 ± 7.56 a | 156.21 ± 10.68 a | 630.15 ± 34.14 b | 1785.81 ± 49.56 a | |
| ASA | 242.55 ± 7.35 a | 12.00 ± 1.08 a | 648.51 ± 21.87 b | 1.98 ± 0.18 a | 175.62 ± 10.65 a | 175.47 ± 9.42 a | 602.97 ± 7.23 ab | 1874.10 ± 36.69 a | |
| 10 days | C | 227.34 ± 4.50 a | 9.93 ± 2.64 a | 678.24 ± 43.26 a | 1.77 ± 0.24 a | 171.51 ± 10.35 a | 168.39 ± 5.67 a | 529.32 ± 12.57 a | 1798.98 ± 55.83 a |
| SA | 235.47 ± 9.99 a | 9.51 ± 1.35 a | 653.10 ± 27.99 a | 2.01 ± 0.21 a | 192.21 ± 5.22 ab | 161.16 ± 6.33 a | 655.95 ± 15.78 b | 1919.13 ± 52.95 a | |
| MeSA | 239.61 ± 6.42 a | 10.83 ± 1.23 a | 726.96 ± 17.25 a | 1.86 ± 0.24 a | 170.46 ± 4.41 a | 168.15 ± 11.37 a | 644.13 ± 10.08 b | 1973.58 ± 32.58 a | |
| ASA | 230.25 ± 7.20 a | 10.77 ± 1.86 a | 655.56 ± 23.43 a | 2.70 ± 0.48 a | 219.03 ± 7.89 b | 170.97 ± 14.22 a | 678.36 ± 35.25 b | 1979.85 ± 79.50 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gačnik, S.; Veberič, R.; Hudina, M.; Koron, D.; Mikulič-Petkovšek, M. Salicylate Treatment Affects Fruit Quality and Also Alters the Composition of Metabolites in Strawberries. Horticulturae 2021, 7, 400. https://doi.org/10.3390/horticulturae7100400
Gačnik S, Veberič R, Hudina M, Koron D, Mikulič-Petkovšek M. Salicylate Treatment Affects Fruit Quality and Also Alters the Composition of Metabolites in Strawberries. Horticulturae. 2021; 7(10):400. https://doi.org/10.3390/horticulturae7100400
Chicago/Turabian StyleGačnik, Saša, Robert Veberič, Metka Hudina, Darinka Koron, and Maja Mikulič-Petkovšek. 2021. "Salicylate Treatment Affects Fruit Quality and Also Alters the Composition of Metabolites in Strawberries" Horticulturae 7, no. 10: 400. https://doi.org/10.3390/horticulturae7100400